Product defects and withdrawal of medicinal products in 2006
Introduction
Number of reported product defects
Reports broken down by source
Types of defects
Counterfeit medicinal products
Deviations from specifications
Withdrawals
Reports compared to previous years
In 2006, there were more reports on product defects compared with 2005. The reports concerned both marketed and non-marketed medicinal products, including magistral medicinal products, which are manufactured according to a doctor's directions.
Introduction
The Danish Medicines Agency must be informed if a company estimates that a product defect may lead to the withdrawal of a medicinal product from the market. This is stipulated in section 30 of Executive Order no. 1242 of 12 December 2005 on manufacturing and import of medicinal products and intermediary products. The Danish Medicines Agency also receives notifications about product defects from foreign medicines agencies via the so-called 'Rapid Alert System'.
The Danish Medicines Agency carefully considers such notifications, which also implies that we assess the gravity of the product defects. Before a decision is made to withdraw a medicinal product, several aspects must be examined, among other things whether the medicinal product is marketed in Denmark, whether it is being clinically tested or dispensed via a special compassionate use permit. Moreover, the medicinal product might have been exported from Denmark .
If a defective medicinal product is on the Danish market, or if it has been exported, we effect a withdrawal in collaboration with the company. It is estimated how far down the chain of distribution the medicinal product is to be withdrawn (wholesaler, pharmacy, consumer), and whether it is relevant to inform other medicines agencies about the withdrawal. Critical product defects are moreover posted as warnings on our website. Where less critical product defects are concerned, we also consider how a withdrawal of the medicinal product from the market would effect the supply of medicine to the Danish people.
Companies must comply with the rules on good manufacturing and distribution practice (GMP and GDP). The rules contribute to minimising potential defects during the manufacturing process and distribution of medicinal products. At inspections, we check whether companies comply with these rules. In addition, the Danish Medicines Agency regularly purchases selected medicinal products to check the packaging information and label and to analyse the medicinal products. During case handling of variations of marketing authorisations, information might uncover defects or changes in medicinal products. Withdrawal of medicinal products can therefore take place both in connection with the Danish Medicines Agency's testing and authorisation of medicinal products and in connection with inspection of companies.
This year, some changes have been made relative to the reports from previous years. In the section Types of defects, we have chosen to put focus on counterfeit medicinal products and specification deviations. We will describe the withdrawals of these two types in particular as the Danish Medicines Agency has received an increasing number of reports about counterfeit medicine in the EU over the past few years. Furthermore, we have also received a large number of reports about medicinal products that do not comply with the specifications.
Number of reported product defects
In 2006, we received a total of 196 reports on product defects concerning medicinal products. Figure 1 shows the number of reports received in the period 2003-2006. In 2006, the number of reports received increased significantly on 2005.
Figure 1. Number of reports on product defects 2003-2006
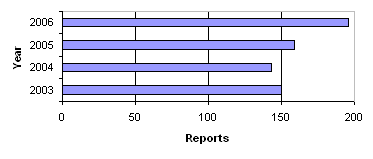
The 196 reports on product defects in 2006 cover:
- Reports on product defects from companies and authorities (> 90 % of the reports)
- Complaints from medicine users
- Discovery of counterfeit medicinal products which are not manufactured by the authorised pharmaceutical manufacturer, and where quality, effect and safety are not documented
- Discovery of dietary supplements/natural remedies containing active substances
The reports concern both marketed and non-marketed medicinal products, including magistral medicinal products, which are manufactured according to a doctor's directions.
Reports broken down by source
Figure 2 shows the 196 reports received in 2006 broken down by source.
Figure 2 . Reports broken down by source
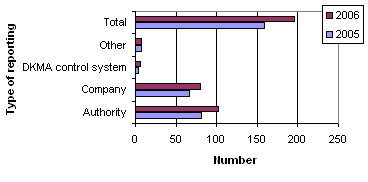
2006 saw an increased number of reports from medicines agencies and manufactures or importers compared with 2005. In 2006, the number of reports in the form of user complaints (category: other) and the Danish Medicines Agency's (DKMA) control/authorisation was roughly the same as in 2005.
Types of defects
When the reports are registered at the Danish Medicines Agency, they are divided into six different types of defects as illustrated by figure 3 below.
Figure 3 . Reports broken down by type of defect
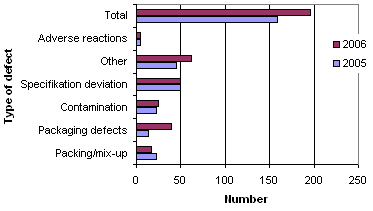
Compared with 2005, 2006 showed fewer defects related to packaging and mix-ups, whereas there was an increase in the number of 'other deviations' (e.g. counterfeit medicinal products) and defects in the packaging information. Defects related to the packaging information often appear in connection with the repackaging of medicinal products. The number of defects related to contamination, deviation from specifications and adverse reactions was roughly the same as in 2005.
Counterfeit medicinal products
The Rapid Alert System has reported an increasing number of warnings about counterfeit medicinal products in the legitimate supply chain in the period 2003-2006, cf. table 1. Nevertheless, we have seen no counterfeit medicinal products in the legitimate supply chain in Denmark so far.
Table 1. Number of withdrawals in the period 2003-2006.
2003 |
2004 |
2005 |
2006 | |
Counterfeit medicinal products |
0 |
2 |
3 |
8 |
Deviations from specifications
Out of the 49 reports about defects concerning deviation from specifications, 18 resulted in withdrawals in Denmark for the following reasons:
- Changed durability (five withdrawals)
- The actual content of the active substance deviated from the labelled active ingredient (five withdrawals)
- Precipitation and crystallisation (three withdrawals)
- Leaks (two withdrawals)
- Change of colour (one withdrawal)
- Lack of control (one withdrawal)
- Change of particle size (one withdrawal)
Withdrawals
Of the total of 196 reports, 28% resulted in withdrawals of medicinal products from the Danish market.
Compared to 2005 and 2004, a proportionately smaller number of reports resulted in withdrawals. Still, there are no major variations compared with 2003, cf. table 2.
Table 2. Number of withdrawals in the period 2002-2006.
2002 |
2003 |
2004 |
2005 |
2006 | |
Number of reports |
192 |
150 |
143 |
160 |
196 |
Number of withdrawals |
72 |
39 |
58 |
58 |
55 |
Withdrawals in percent |
38% |
26% |
41% |
36% |
28% |
Table 3. Reports resulting in withdrawals broken down by source in 2005-2006
Number in 2005 |
Number in 2006 | |
Company |
44 |
39 |
Foreign agency |
11 |
10 |
Danish Medicines Agency's control system |
2 |
2 |
Other |
1 |
4 |
Total |
58 |
55 |
Table 3 shows a minor change between 2005 and 2006. There were vaguely fewer reports submitted by companies in 2006 compared with 2005, whereas the number of reports from foreign agencies and the Danish Medicines Agency's control system was on a par with 2005. In conclusion, the distribution of reports has not changed much since 2005 in general.
39 withdrawals were based on reports from companies, and they were primarily caused by product defects in the form of specification deviations. Apart from specification deviations, there were also other defects related to packaging information, contamination or precipitation, etc.
Three of the ten withdrawals, which were launched after reports from foreign authorities, were due to specification deviations, two were due to contamination and one was due to defects related to packaging information. The remaining four were due to other defects.
The two withdrawals initiated by the Danish Medicines Agency were occasioned by specification deviations.
Reports compared to previous years
In 2006, there were more reports on product defects compared with the preceding three years, cf. figure 1. In 2006, an increasing number of reports were initiated by other medicines agencies and manufacturers or importers compared with previous years.
The number of withdrawals of defective medicinal products manufactured in Denmark and placed on the Danish market and export markets is roughly the same as last year. These withdrawals primarily concern specification deviations and defects in respect of labelling, package or package leaflets.
The Danish Medicines Agency, 26 July 2007
