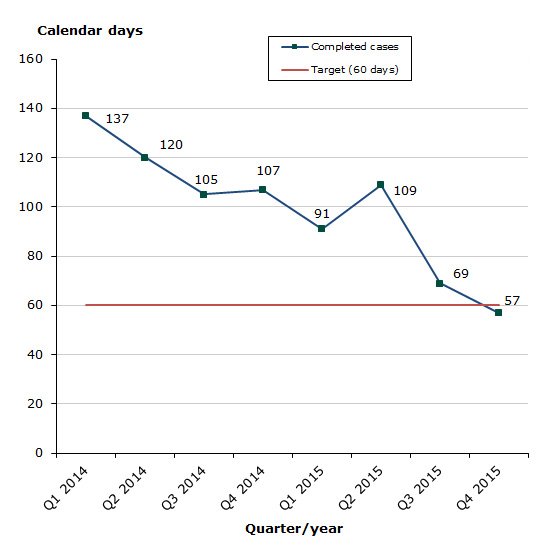
Assessment times for authorisation of parallel import of medicines 2015
Status for 2015 as at end-December 2015: The assessment time has significantly improved since Q1 2014 and in 2015 we have focused on completing a number of cases that have given rise to fundamental reflections concerning the establishment af the necessary identity between the parallel imported product and the directly imported medicinal product. In addition, it should be mentioned that the average number of completed cases per quarter has increased continuously: 120 cases in 2013, 190 cases in 2014 and 174 cases in 2015.
76 % of the cases finished within the performance requirement's maximum of 60 days (average assessment time is 86 days):
- Human medicines: 77 % of the cases finished within the performance requirement's maximum of 60 days (82 days on average).
- Veterinary medicines: 0 % of the cases finished within the performance requirement's maximum of 60 days (347 days on average). Only a small amount of cases were included in the measurement (11 cases)
Chart 1: Average assessment times for parallel import
See table 1-3 for parallel import
The assessment times are measured against the target time of 60 days, i.e. from the date when we start reviewing the application until we issue the marketing authorisation. The assessment times are net times, which means that they do not include any time periods during which we await a reply from the applicant or the export country.
