Assessment times for issuance of marketing authorisations in Concerned Member State cases
Under the mutual recognition procedure and the decentralised procedure, we have 30 days to issue a marketing authorisation from the date when we receive an acceptable Danish summary of product characteristics (SmPC) or notification that the medicinal product is not to be marketed in Denmark and therefore a marketing authorization must be issued without Danish product characteristics (SmPC).
Status in 2024 as at end-December 2024: 44 % of the cases finished within the performance requirement's maximum of 30 days (94 days on average):
- Human medicines: 39 % of the cases finished within the performance requirement's maximum of 30 days (101 days on average).
- Veterinary medicines: 78 % of the cases finished within the performance requirement's maximum of 30 days (41 days on average).
Due to the Covid-19 pandemic, the Danish Medicines Agency did not prioritized the assessments times. We therefore build up a backlog of cases which we have reduced in 2023 and 2024. From the 1st of January 2025 it is expected to reach the target time of 30 days for new cases, while the last delayed cases would be completed on an ongoing basis at the beginning of the year. It is expected that the assessment times will reach 95% compliance for all cases completed in the 2nd half of 2025.
Chart 1: Average assessment times for issuance of marketing authorisations with Denmark as Concerned Member State (CMS)
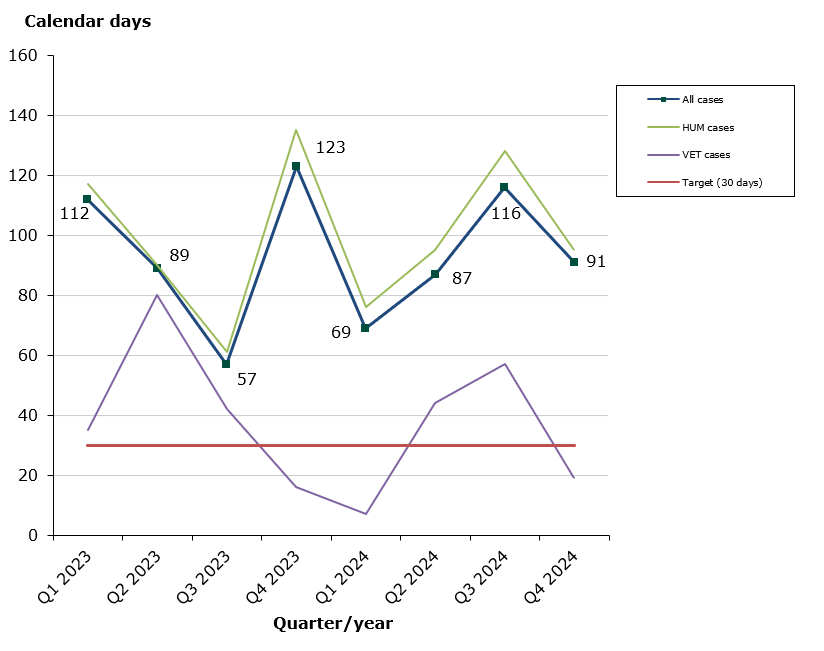
The Danish Medicines Agency has established a best practice agreement on the issuance of marketing authorisations in connection with MRP/DCP applications where Denmark is Concerned Member State. According to this agreement, the maximum assessment time allowed is 30 days from the date on which we receive an acceptable Danish version of the authorised SmPC or notification that the medicinal product is not to be marketed in Denmark until issuance of a marketing authorisation.
For further information, please contact Send an email.
