Assessment times for national variations 2013
The assessment times are divided respectively in human and veterinary medicines. We calculate the total assessment time for the three types of variations:
Type IA variations
Type IB variations
Type II variations
The assessment times reported are broken down by type IA, IB and II variations and calculated in the following way:
Assessment times for type IA variations: The assessment time is measured against the target time of 20 days from the date on which we receive the application until the date of determination (grant or refusal).
Assessment times for type IB variations: The assessment time is measured against the target time of 95 days from the date on which we receive the application until the date of determination (grant or refusal), excluding any clock-stop days.
Assessment times for type II variations: The assessment time is measured against the target time of 185 days from the date on which we receive the application until the date of determination (grant or refusal), excluding any clock-stop days.
For Q4 2013, we make the following observations:
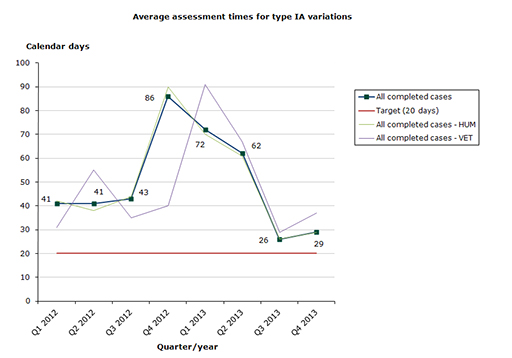
Type IA variations: It remains problematic to satisfy the performance measure of 20 days for type IA variations.
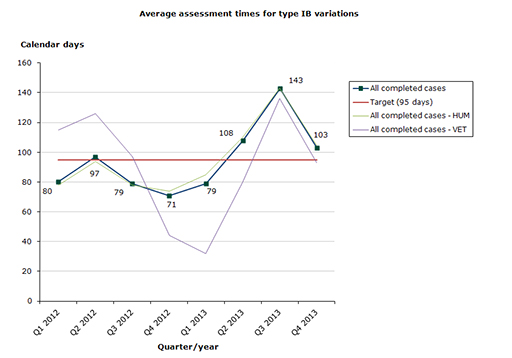
Type IB variations: Chemical variations, in particular, have a too long start-up phase on average. The share of variations assessed within the target time is around 60 % since 2011.
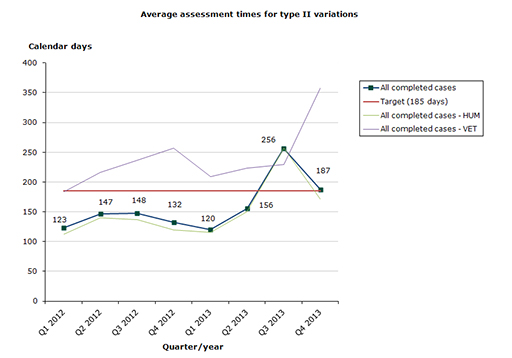
Type II variations: The average assessment time has been satisfactory for the past two years. Approx. 70-80 % of variations were assessed within the target time in 2011, 2012 and first 2 quarters of 2013. In the 3rd and4th quarter of 2013 only respectively 25 % and 61 % of the variations are assessed within the target time, due to the completion of a large amount of quality variations, that have been delayed in the start-up phase.
Generally, it can be said for both type IB and II variations that we previously have had difficulty meeting the performance requirements in the biological field compared to the chemical field. This is no longer the case. Future delays are expected in the chemical field. The reason for this is due to many chemical variations and prioritization of other approval procedures. For the next 2 years, we will be working on removing the accumulated amount of type IB and II variations.
Type IA variations
Assessment time in 2013 for type IA variations as at end-December 2013: 37 % of the cases finished within the performance requirement's maximum of 20 days (49 days on average):
- Human medicines: 37 % of the cases finished within the performance requirement's maximum of 20 days (49 days on average).
- Veterinary medicines: 41 % of the cases finished within the performance requirement's maximum of 20 days (62 days on average).
Chart 1. Assessment times for type IA variations
See table 1-3 for type IA variations
Type IB variations
Assessment time in 2013 for type IB variations as at end-December 2013: 57 % of the cases finished within the performance requirement's maximum of 95 days (112 days on average):
- Human medicines: 56 % of the cases finished within the performance requirement's maximum of 95 days (115 days on average)
- Veterinary medicines: 68 % of the cases finished within the performance requirement's maximum of 95 days (83 days on average)
Chart 2. Assessment times for type IB variations
See table 4-6 for type IB variations
Type II variations
Assessment time in 2013 for type II variations as at end-December 2013: 52 % of the cases finished within the performance requirement's maximum of 185 days (192 days on average).
- Human medicines: 53 % of the cases finished within the performance requirement's maximum of 185 days (188 days on average).
- Veterinary medicines: 31 % of the cases finished within the performance requirement's maximum of 185 days (263 days on average).
Chart 3. Assessment times for type II variations in the start-up phase