Assessment times for new national applications for the licensing of medicinal products 2013
The assessment times are divided respectively in human and veterinary medicines. The assessment time of new national applications for the licensing of medicinal products, are calculated in the following way:
Completed cases
- Total assessment time
- Assessment time of the start-up phase.
- Assessment time of remaining phases
Initiated cases with a completed start-up phase
- Assessment times of these cases
For Q4 2013, we make the following observations:
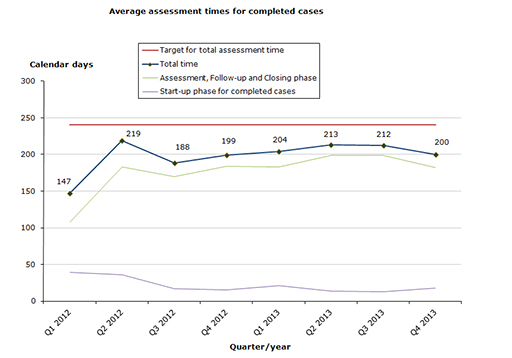
In 2013, we have completed 30 cases, of which 28 cases (93 %) where completed within the performance requirement of 240 days. In 2012, 89 % of all cases where finished within the performance requirement.
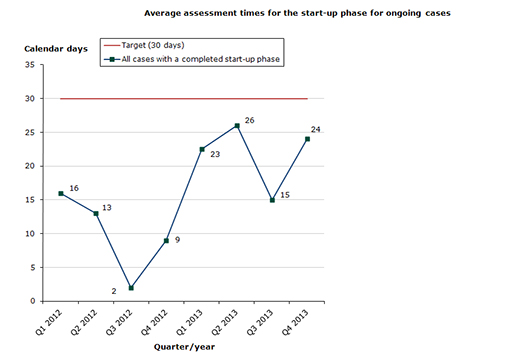
While focusing on the total assessment time, we make every effort to meet the maximum time allowed in the start-up phase, and Chart 2 below shows the assessment time for cases with a completed start-up phase (i.e. the assessment time is measured from the close of the start-up phase).
Total assessment times for completed cases
Status for 2013 as at end-December 2013: 30 cases have been completed, 20 cases for human medicines and 10 cases for veterinary medicines.
Total for human and veterinary medicines
- 93 % of the cases finished within the performance requirement's maximum of 240 days (207 days on average).
- 83 % of the cases finished within the performance requirement's maximum of 30 days in the start-up phase (17 days on average).
- 80 % of the cases finished within the performance requirement's maximum of 210 days in the assessment and closing phases (190 days on average).
Human medicines
- 90 % of the cases finished within the performance requirement's maximum of 240 days (212 days on average).
- 85 % of the cases finished within the performance requirement's maximum of 30 days in the start-up phase (15 days on average).
- 80 % of the cases finished within the performance requirement's maximum of 210 days in the assessment and closing phases (197 days on average).
Veterinary medicines
- 100 % of the cases finished within the performance requirement's maximum of 240 days (197 days on average).
- 80 % of the cases finished within the performance requirement's maximum of 30 days in the start-up phase (20 days on average).
- 80 % of the cases finished within the performance requirement's maximum of 210 days in the assessment and closing phases (177 days on average).
Chart 1. Assessment times for completed cases
See table 1-9 for completed cases
The total assessment time is measured against the target time of 240 days for 2013 from the date on which we receive the application until the date of determination (grant or refusal), excluding any clock-stop days.
The assessment time in the start-up phase is measured against the target time of 30 days for 2013 from the date when we receive the application until the procedure start date (day 0).
The assessment time for the remaining phases is measured against the target time of 210 days for 2013 from the procedure start date (day 0) until the date of determination (grant or refusal), excluding any clock-stop days.
Assessment times for initiated cases
Status for 2013 as at end-December 2013: 20 out of 25 cases (80 %) finished within the performance requirement's maximum of 30 days in the start-up phase (22 days on average):
- Human medicines: 9 out of 11 cases (82 %) finished within the performance requirement's maximum of 30 days in the start-up phase (25 days on average).
- Veterinary medicines: 11 out of 14 cases (79 %) finished within the performance requirement's maximum of 30 days in the start-up phase (19 days on average).
Chart 2. Assessment times for initiated procedures
View table 10-12 – cases with an initiated procedure
The assessment time is measured against the target time of 30 days for 2013 from the date when we receive the application until the procedure start date (day 0).