Assessment times for new national applications for the licensing of medicinal products in 2024
The assessment times are divided respectively in human and veterinary medicines. The assessment time of new national applications for the licensing of medicinal products, are calculated in the following way:
Completed cases
- Total assessment time
- Assessment time of the start-up phase.
- Assessment time of remaining phases
- Initiated cases with a completed start-up phase
- Assessment times of these cases
For Q4 2024, we make the following observations:
In 2024, we have completed 20 cases, of which 11 cases (55 %) were completed within the performance requirement of 240 days. In 2023, 33 % of all cases where finished within the performance requirement. The completed cases in 2023 and 2024 where received during the Covid-19 pandemic in 2020-2022. Due to the Covid-19 pandemic, the Danish Medicines Agency did not prioritize the assessments times. The changed prioritization takes effect in the assessment times and will therefore affect compliance until the cases are completed.
While focusing on the total assessment time, we make every effort to meet the maximum time allowed in the start-up phase, and Chart 2 below shows the assessment time for cases with a completed start-up phase (i.e. the assessment time is measured from the close of the start-up phase).
Total assessment times for completed cases
Status for 2024 as at end-December 2024: 20 cases for medicine have been completed, all cases for human medicines.
Total for human medicines
- 55 % of the cases finished within the performance requirement's maximum of 240 days (271 days on average).
- 80 % of the cases finished within the performance requirement's maximum of 30 days in the start-up phase (21 days on average).
- 25 % of the cases finished within the performance requirement's maximum of 210 days in the assessment and closing phases (250 days on average).
Chart 1. Assessment times for completed cases
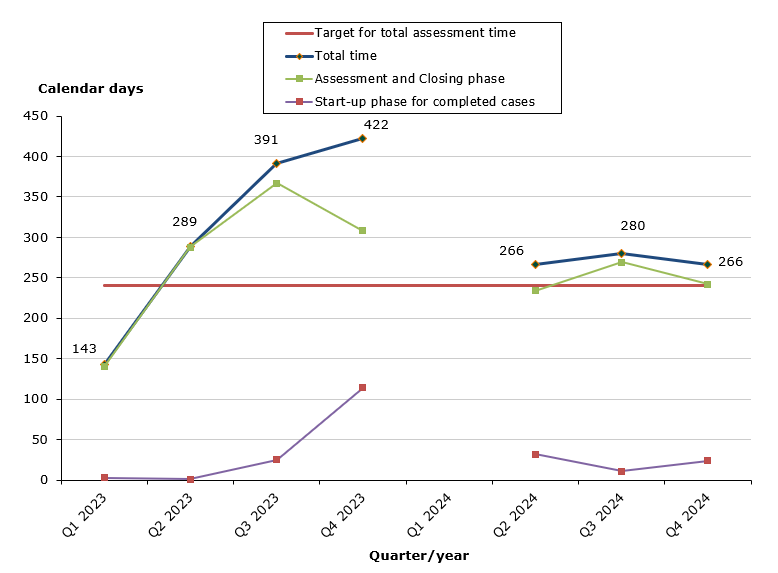
There where no completed cases in Q4 2022 and Q1 2024
See table 1-12 for completed cases
The total assessment time is measured against the target time of 240 days for 2024 from the date on which we receive the application until the date of determination (grant or refusal), excluding any clock-stop days.
The assessment time in the start-up phase is measured against the target time of 30 days for 2024 from the date when we receive the application until the procedure start date (day 0).
The assessment time for the remaining phases is measured against the target time of 210 days for 2024 from the procedure start date (day 0) until the date of determination (grant or refusal), excluding any clock-stop days.
Assessment times for initiated cases
Status for 2024 as at end-December 2024:
-
- Total for human and veterinary medicines: 50 % of the cases finished within the performance requirement's maximum of 30 days (40 days on average).
- Human medicines: 57 % of the cases finished within the performance requirement's maximum of 30 days (36 days on average).
- Veterinary medicines: 0 % of the cases finished within the performance requirement's maximum of 30 days (67 days on average).
Chart 2. Assessment times for initiated procedures
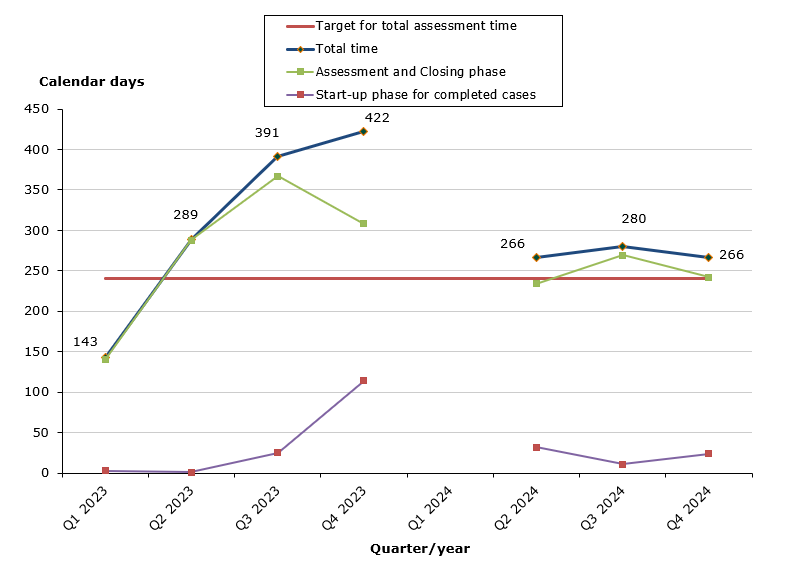
There where no initiated cases in Q2 2023
View table 10-12 – cases with an initiated procedure
The assessment time is measured against the target time of 30 days for 2024 from the date when we receive the application until the procedure start date (day 0).
For further information, please contact Send an email.
