Centralised authorisation procedure 2024
The Danish share of assignments in the European pharmaceutical collaboration
The Danish Medicines Agency should have the greatest possible influence on decisions regarding new medicinal products and thereby the greatest possible influence on the future development of the European market for medicinal products.
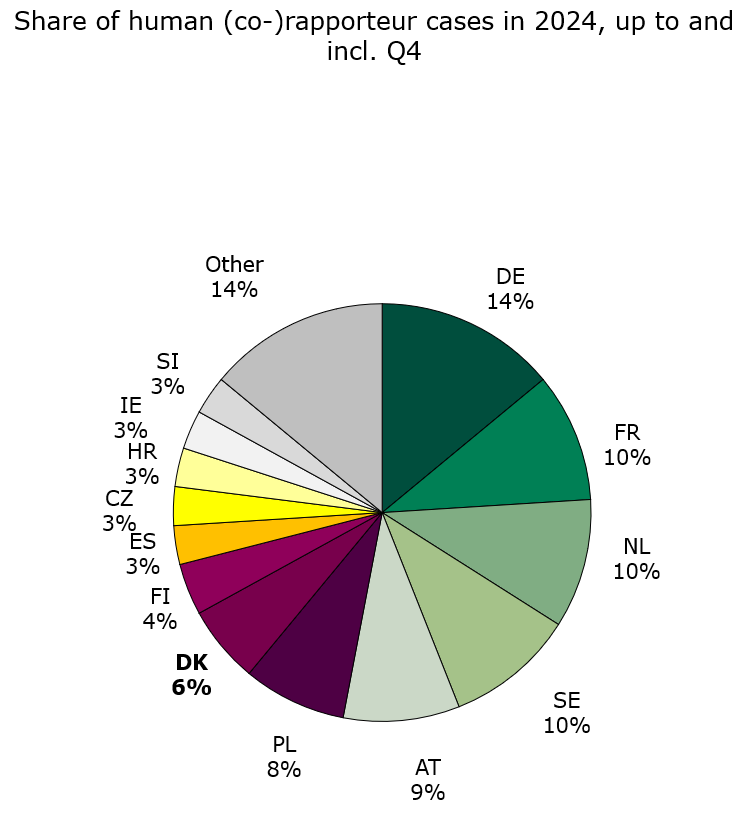
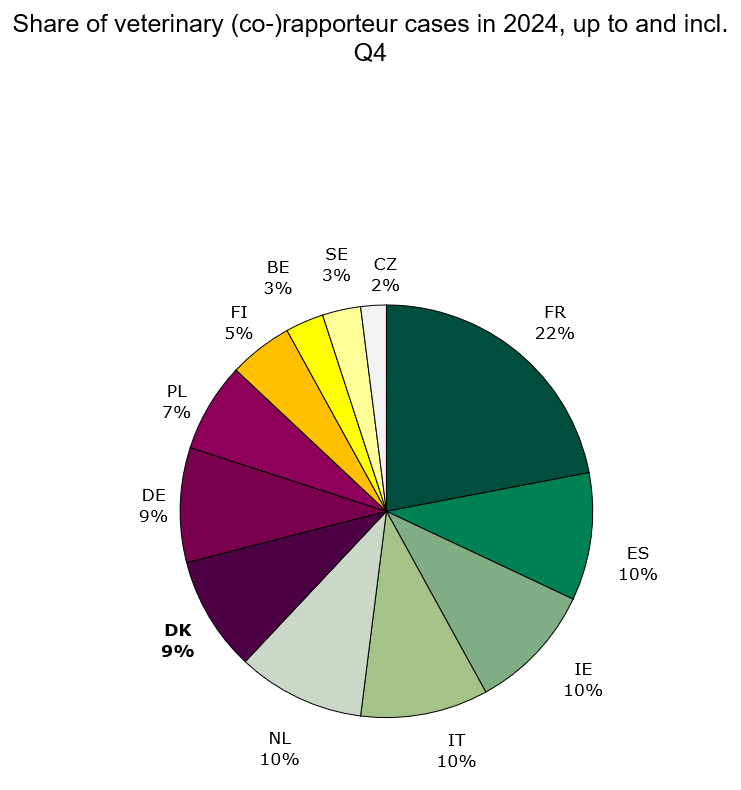
Status for 2024 up to and including 4th quarter: Denmark was assigned 14 human rapporteur/co-rapporteur cases and 6 veterinary rapporteur/co-rapporteur cases, which is respectively 6 % and 9 % of all assigned central procedure cases in 2024.
Figure 1: Amount of assigned (co)rapporteurships in the EU in 2024 up to and including 4th quarter
Figure 2. Share of human Rap/Co-rap cases
Table 1: Assigned human Rapporteur/co-rapporteur cases
|
Number of human procedures |
2023 |
2024 |
||
|
Cases with DK as Rap/Co-rap |
Total amount of cases |
Cases with DK as Rap/Co-rap |
Total amount of cases |
|
|
1st quarter |
4 |
53 |
6 |
75 |
|
2nd quarter |
5 |
49 |
4 |
50 |
|
3rd quarter |
4 |
50 |
2 |
56 |
|
4th quarter |
3 |
70 |
2 |
54 |
|
Total |
16 |
222 |
14 |
235 |
Figure 3. Share of veterinary Rap/Co-rap cases
Table 2: Assigned veterinary Rapporteur cases/co-rapporteur cases
|
Number of veterinary procedures |
2023 |
2024 |
||
|
Cases with DK as Rap/Co-rap |
Total amount of cases |
Cases with DK as Rap/Co-rap |
Total amount of cases |
|
|
1st quarter |
0 |
12 |
3 |
16 |
|
2nd quarter |
1 |
16 |
0 |
10 |
|
3rd quarter |
0 |
10 |
2 |
20 |
|
4th quarter |
2 |
16 |
1 |
22 |
|
Total |
3 |
54 |
6 |
68 |
Please contact Send an email for further information.