Product defects and withdrawal of medicinal products in 2005
Introduction
Number of reported product defects
Distribution of reports into sources
Types of defects
Withdrawals
Reports compared to previous years
In 2005, there was almost the same number of reports on product defects as in 2004. The reports comprised both marketed and non-marketed medicinal products, including magistral medicinal products, which are manufactured according to a doctor’s prescription.
Introduction
The Danish Medicines Agency must be informed if a company estimates that a product defect may lead to the withdrawal of a medicinal product from the market. This is stipulated in section 30 of Executive Order no. 1242 on manufacturing and import of medicinal products and intermediary products.
The Danish Medicines Agency carefully considers such notifications, hereunder the nature of the product defect is assessed. If it is decided that a medicinal product is to be withdrawn, several aspects must be examined, among other things whether the medicinal product is marketed in Denmark , whether it is being clinically tested or dispensed via a special compassionate use permit. Moreover, the medicinal product might have been exported from Denmark .
If a defective medicinal product is on the Danish market, or if it has been exported, we effect a withdrawal in collaboration with the company. It is estimated how far down the chain of distribution the medicinal product is to be withdrawn (wholesaler, pharmacy, consumer), and whether it is relevant to inform other pharmaceutical authorities about the withdrawal. As regards serious product defects, we usually post warnings on our website.
Companies must comply with the rules on good manufacturing and distribution practice that contribute to minimising the possibility for defects during the manufacture and distribution of medicinal products. At inspections, we control whether companies comply with these rules. Furthermore, on an ongoing basis the Danish Medicines Agency purchases selected medicinal products, controls the packaging and label, and analyses the medicinal products. During case handling of variations of marketing authorisations, information might uncover defects or changes in medicinal products. Withdrawal of medicinal products can therefore take place both in connection with the Danish Medicines Agency’s control and authorisation of medicinal products and in connection with inspection of companies.
The Danish Medicines Agency also receives quite a lot of reports from pharmaceutical authorities in other countries concerning withdrawals in the countries in question. According to mutual agreements, we receive information on the most serious defects as well as on defects concerning medicinal products distributed to Denmark . In addition, we receive complaints from medicine users.
This year, some changes have been made relative to the reports from previous years. First and foremost, it has been attempted to make a report that is easier to read with graphs and more detailed information.
Under the section on types of defects, we have chosen to focus on contamination problems and packaging defects as well as to describe the withdrawals in these cases. The reason for this is that in these cases in particular, it is possible to make an effort to reduce the number of defects.
Number of reported product defects
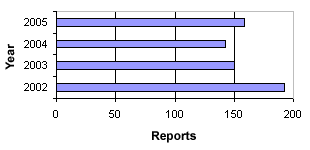
In 2005, we received a total of 160 reports on product defects concerning medicinal products. The number of reports received in the period 2002-2005 can be seen in figure 1. In 2005, there was a slight increase in the number of reports compared to 2004.
Figure 1. Number of reports on product defects 2002-2005
The 160 reports on product defects in 2005 cover:
- Reports on product defects from companies and authorities (> 90 % of the reports)
- Complaints from medicine users
- Discovery of counterfeit medicinal products, which are not manufactured by the authorised pharmaceutical manufacturer, and where quality, effect and safety are not documented
- Discovery of dietary supplements/natural remedies containing active substances
The reports concern both marketed and non-marketed medicinal products, hereunder magistral medicinal products, which are manufactured according to a doctor’s directions.
Distribution of reports into sources
Figure 2 below shows the distribution of sources for the 160 reports in 2005.
Figure 2. Distribution into sources
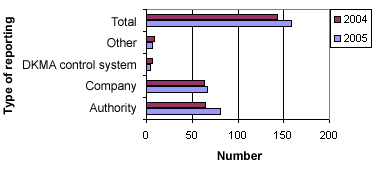
In 2005, there were fewer reports in the form of user complaints (category: Other) and fewer cases that were initiated by the Danish Medicines Agency’s (DKMA) control/authorisation than in 2004. However, the number of reports from other pharmaceutical authorities and from manufacturers or importers has increased.
Types of defects
When the reports are registered at the Danish Medicines Agency, they are divided into six different types of defects. Figure 3 below shows the distribution.
Figure 3. Distribution into types of defects
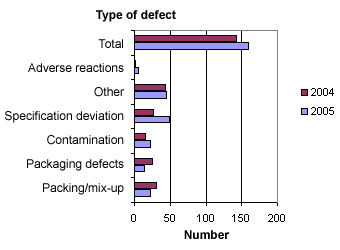
Compared to 2004, fewer defects relating to packing, mix-ups and packaging were observed in 2005, whereas there were more defects relating to contamination and deviation from specifications. The number of ‘other deviations’ (e.g. counterfeit medicinal products) and withdrawal cases concerning adverse reactions was more or less the same as in 2004.
Contamination
Out of 22 reports on contaminated medicinal products, four reports led to withdrawal in Denmark . In the 18 remaining cases, no withdrawals were effected in Denmark , as the medicinal products were not sold in Denmark , which was the most frequent reason. In one case, it was estimated that it was an isolated occurrence and therefore it was not deemed necessary to make a withdrawal. Four of the 22 reports related to medicinal products or intermediate products manufactured in Denmark .
The four cases in which withdrawals were effected in Denmark were based on the following causes:
- Fungal growth in the product
- Contamination of the active substance with ethylene glycol during vacuum drying
- Cross-contamination with another active substance
- Risk of virus content in blood plasma for the manufacture of medicinal products
Packaging
There were 14 reports on defective packaging, and five withdrawals were effected in Denmark . The five withdrawals were distributed on the following causes:
- Wrong expiry date indicated (two withdrawals)
- Wrong packaging material (packaging with English writing)
- Wrong indication of strength
- Defective infusion containers
Withdrawals
Of the total of 160 reports, 37 % resulted in withdrawals of medicinal products from the Danish market or the export market. In other words, the medicinal products concerned were either manufactured in or imported into Denmark .
Compared to 2004, this is a smaller share of the total number of reports resulting in withdrawal, but there have been no great deviations since 2001, see table 1.Table 1. Number of withdrawals in the period 2002-2005.
|
2001 |
2002 |
2003 |
2004 |
2005 |
Number of reports |
166 |
192 |
150 |
143 |
160 |
Number of withdrawals |
59 |
72 |
39 |
58 |
58 |
Withdrawals in per cent |
36% |
38% |
26% |
41% |
36% |
Table 2. Distribution of reports resulting in withdrawals in 2004-2005.
|
Total in 2004 |
Total in 2005 |
Company |
46 |
44 |
Foreign authority |
5 |
11 |
The Danish Medicines Agency’s control system |
6 |
2 |
Other |
1 |
1 |
Total |
58 |
58 |
Table 2 shows that there were minor changes in 2005 compared to 2004. There was a slightly higher number of reports from foreign authorities in 2005 than in 2004, whereas there were fewer reports from the Danish Medicines Agency’s control system in 2005 than in 2004. It can be concluded that the overall distribution has not changed since 2004.
44 withdrawals were based on reports from companies and they were primarily caused by product defects in the form of specification deviations. Examples of specification deviations are formation of peroxide in the product and discolouration in reconstitution. Apart from specification deviations, there were also other defects, such as storage at the wrong temperature, defective packaging, withdrawal due to adverse reactions, changed dispense terms, mix-ups, contamination or precipitation.
Five of the 11 withdrawals, which were launched after reports from foreign authorities, were due to specification deviations, one was due to adverse reactions (withdrawn for replacement of package leaflets) and the remaining five withdrawals were due to other defects, such as wrong labelling or lack of updates of the package leaflet.
The causes for the two withdrawals initiated by the Danish Medicines Agency were lack of compliance with specifications and sale of a product as a dietary supplement where the product was regarded as a medicinal product.
Reports compared to previous years
In 2005, there was a slightly higher number of reports on product defects than in 2003 and 2004, but still fewer than in 2002, cf. figure 1. In 2005, fewer cases were initiated based on the Danish Medicines Agency’s control/authorisation and user complaints. However, there were more reports from other pharmaceutical authorities and from manufacturers or importers than previous years.
The number of withdrawals of defective medicinal products in Denmark and export countries to which medicinal products manufactured in Denmark have been sent is roughly the same as last year. These withdrawals are primarily due to specification deviations and defective packing, label, packaging or package leaflets.
The Danish Medicines Agency, 15 November 2006
