Assessment times for national variations in 2022
Variations for human medicinal products are divided in three types of variations:
Type IA variations and VNRA
Type IB variations and VRA reduced
Type II variations and standard/extended
Variations for the veterinary medicinal products applied for before 28th of January 2022 are also divided into Type IA / IB / II, while the variations applied for after 28th of January 2022 are divided into VNRA, VRA reduced, VRA standard and VRA extended, according to the new regulation for veterinary medicinal products . Types IA / IB / II and VNRA / VRA for the veterinary medicinal products are broken down according to the target assessment times:
Type IA and VNRA variations: 30 days
Type IB and VRA reduced: 60 days
Type II and VRA standard/extended: 150 days
The assessment times reported are calculated in the following way:
Assessment times for type IA and VNRA variations: The assessment time is measured against the target time of 30 days from the date on which we receive the application until the date of determination (grant or refusal).
Assessment times for type IB and VRA reduced variations: The assessment time is measured against the target time of 60 days from the start of procedure until the date of determination (grant or refusal), excluding any clock-stop days.
Assessment times for type II VRA standard/extended variations: The assessment time is measured against the target time of 150 days from the date on which we receive the application until the date of determination (grant or refusal), excluding any clock-stop days.
For Q4 2022, we make the following observations for variations for human medicinal products:
Type IA variations: In 2022, the share of variations assessed within the target time has been 74 %, while in 2021 was 86 %.
Type IB variations: In 2022, the share of variations assessed within the target time has been 72 %, while in 2021 was 82 %.
Type II variations: In 2022, the share of variations assessed within the target time has been 52 %, while in 2021 was 57 %.
For Q4 2022, we make the following observations for variations for veterinary medicinal products:
Type IA and VNRA variations: In 2022, the share of variations assessed within the target time has been 96 %, while in 2021 was 88 %.
Type IB and VRA reduced variations: In 2022, the share of variations assessed within the target time has been 55 %, while in 2021 was 78 %.
Type II and VRA standard/extended variations: In 2022, the share of variations assessed within the target time has been 20 %, while in 2021 was 29 %.
Due to the Covid-19 pandemic, the Danish Medicines Agency has not prioritized the assessments times. It is expected that the assessments times will reach 95% compliance in 2024.
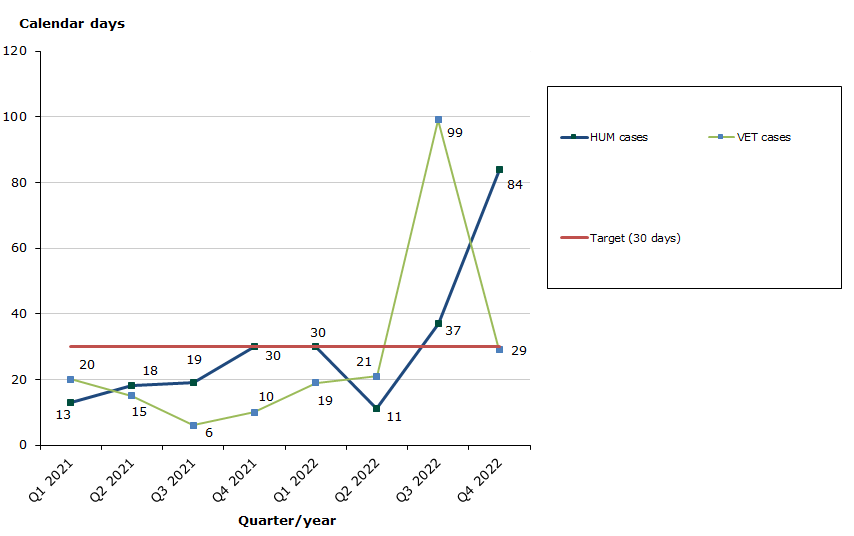
Type IA and VNRA variations
Assessment time for human medicinal products in 2022 for type IA variations as at end-December 2022: 74 % of the cases finished within the performance requirement's maximum of 30 days (49 days on average).
Assessment time for veterinary medicinal products in 2022 for type IA and VNRA variations as at end-December 2022:88 % of the cases finished within the performance requirement's maximum of 30 days (34 days on average).
Chart 1. Assessment times for type IA and VNRA variations
See table 1-2 for type IA variations
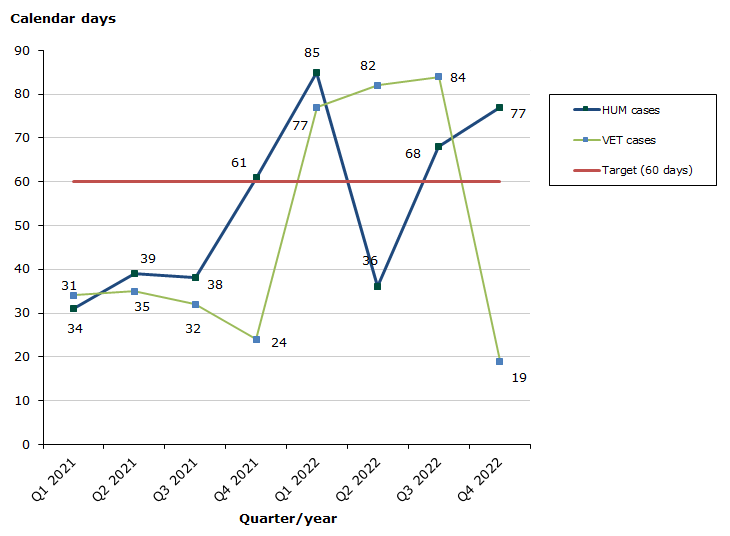
Type IB and VRA reduced variations
Assessment time for human medicinal products in 2022 for type IB variations as at end-December 2022: 72 % of the cases finished within the performance requirement's maximum of 60 days (66 days on average):
Assessment time for veterinary medicinal products in 2022 for type IB and VRA reduced variations as at end-December 2022: 78 % of the cases finished within the performance requirement's maximum of 60 days (48 days on average):
Chart 2. Assessment times for type IB and VRA reduced variations
See table 3-4 for type IB variations
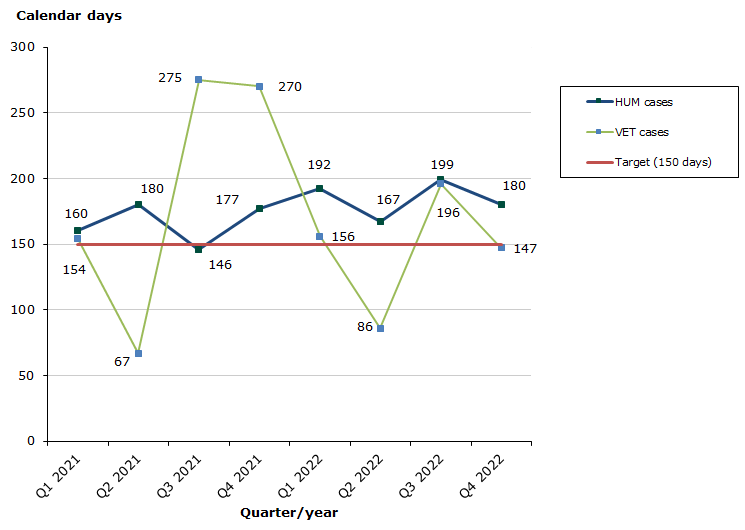
Type II and VRA standard/extended variations
Assessment time for human medicinal products in 2022 for type II variations as at end-December 2022: 52 % of the cases finished within the performance requirement's maximum of 150 days (183 days on average).
Assessment time for veterinary medicinal products in 2022 for type II variations as at end-December 2022: 29 % of the cases finished within the performance requirement's maximum of 150 days (172 days on average).
Chart 3. Assessment times for type II and VRA standard/extended variations
See table 5-6 for type II variations
For further information, please contact godkendelse@dkma.dk.