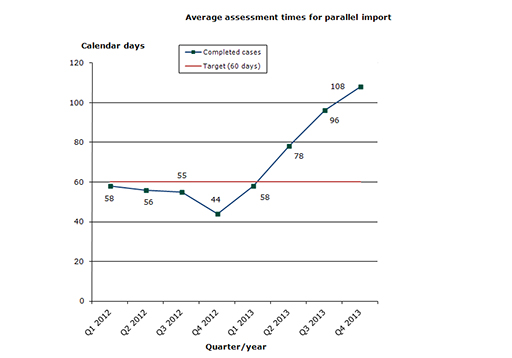
Assessment times for authorisation of parallel import of medicines 2013
Status for 2013 as at end-December 2013:
The share of cases that meet the performance requirements is unfortunately less than usual in this field. This is due to a temporary decline in the resources assigned within this field. We therefore expect that the temporary deterioration will be rectified during 2014 (Q3/Q4).
46 % of the cases finished within the performance requirement's maximum of 60 days (average assessment time is 85 days).
- Human medicines: 46 % of the cases finished within the performance requirement's maximum of 60 days (85 days on average).
- Veterinary medicines: 0 % of the cases finished within the performance requirement's maximum of 60 days (86 days on average). Only 4 veterinary parallel import cases are completed in 2013.
Chart 1: Average assessment times for parallel import
See table 1-3 for parallel import
The assessment times are measured against the target time of 60 days, i.e. from the date when we start reviewing the application until we issue the marketing authorisation. The assessment times are net times, which means that they do not include any time periods during which we await a reply from the applicant or the export country.