Assessment times for authorisation of parallel import of medicines 2019
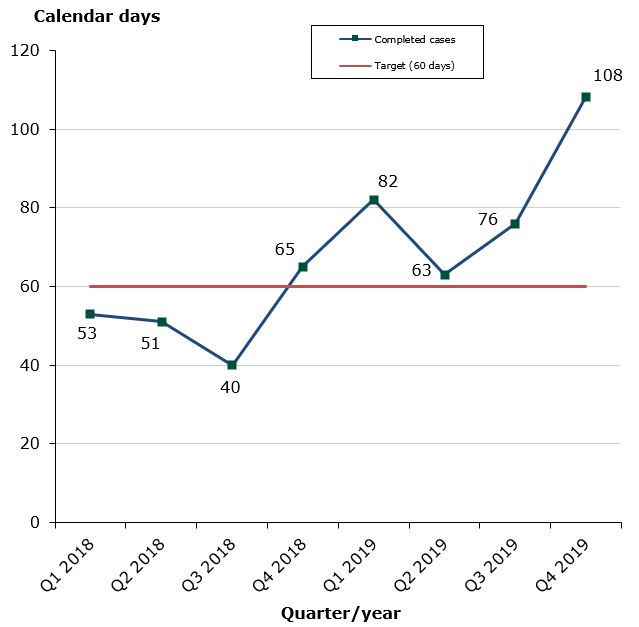
Status for 2019 as at end-December 2019: In 2019, the share of cases assessed within the target time has been 49 %, while in 2018 was 87 %. The low compliance with the assessment times in 2019 is due to a reduction of the staff in 2018, which led to a case accumulation of parallel import applications. In addition, we have received 200 more cases than expected during the year.
49 % of the cases finished within the performance requirement's maximum of 60 days (average assessment time is 81 days):
- Human medicines: 49 % of the cases finished within the performance requirement's maximum of 60 days (81 days on average).
- Veterinary medicines: 50% of the cases finished within the performance requirement's maximum of 58 days.
Chart 1: Average assessment times for parallel import
See table 1-3 for parallel import
The assessment times are measured against the target time of 60 days, i.e. from the date when we start reviewing the application until we issue the marketing authorisation. The assessment times are net times, which means that they do not include any time periods during which we await a reply from the applicant or the export country.
