Assessment times for national variations 2015
The assessment times are divided respectively in human and veterinary medicines. We calculate the total assessment time for the three types of variations:
Type IA variations
Type IB variations
Type II variations
On 1 January 2014, the requirements for the national assessment times changed, in order to align the requirements with respect to variations through European procedures (MRP and centralised procedure).
The assessment times reported are broken down by type IA, IB and II variations and calculated in the following way, for variations completed after the 1st of January 2014:
Assessment times for type IA variations: The assessment time is measured against the target time of 30 days from the date on which we receive the application until the date of determination (grant or refusal). For variations completed before the 1st of January, the target time was 20 days.
Assessment times for type IB variations: The assessment time is measured against the target time of 60 days from the start of procedure until the date of determination (grant or refusal), excluding any clock-stop days. For variations completed before the 1st of January, the target time was 95 days, from the date on which we received the application until the date of determination.
Assessment times for type II variations: The assessment time is measured against the target time of 150 days from the date on which we receive the application until the date of determination (grant or refusal), excluding any clock-stop days. For variations completed before the 1st of January, the target time was 185 days.
Applications received before the new regulations where implemented, are therefore measured according to the new requirements.
For Q4 2015, we make the following observations:
Type IA variations: In 2015, the share of variations assessed within the target time has been 77 %, while in 2014 was 71 %.
Type IB variations: In 2015, the share of variations assessed within the target time has been 85 %, while in 2014 was 78 %.
Type II variations: Since 2013 the share of cases within the target time has been around 50 %, due to the completion of a large amount of quality variations, that were delayed in the start-up phase. In 2015, the share of variations assessed within the target time has been 61 %, while in 2014 was 44 %.
Generally, it can be said for both type IB and II variations that we previously have had difficulty meeting the performance requirements in the biological field compared to the chemical field. This is no longer the case. Instead, we currently have challenges in the chemical field for type II variations. The reason for this is due to the receipt of many chemical variations and the need to prioritize other approval procedures. We are working on removing the accumulated amount of type II variations, and in 2015, we have reduced the amount of months that the oldest type II variations have to wait before start of procedure, from 23 months to 15 months.
Type IA variations
Assessment time in 2015 for type IA variations as at end-December 2015: 77 % of the cases finished within the performance requirement's maximum of 30 days (36 days on average):
- Human medicines: 76 % of the cases finished within the performance requirement's maximum of 30 days (38 days on average).
- Veterinary medicines: 91 % of the cases finished within the performance requirement's maximum of 30 days (22 days on average).
Chart 1. Assessment times for type IA variations
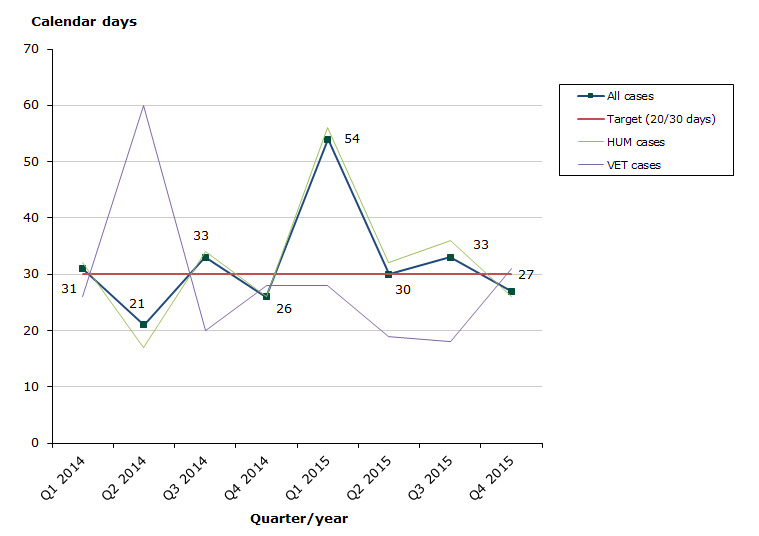
See table 1-3 for type IA variations
Type IB variations
Assessment time in 2015 for type IB variations as at end-December 2015: 85 % of the cases finished within the performance requirement's maximum of 60 days (45 days on average):
- Human medicines: 85 % of the cases finished within the performance requirement's maximum of 60 days (45 days on average)
- Veterinary medicines: 85 % of the cases finished within the performance requirement's maximum of 60 days (44 days on average)
Chart 2. Assessment times for type IB variations
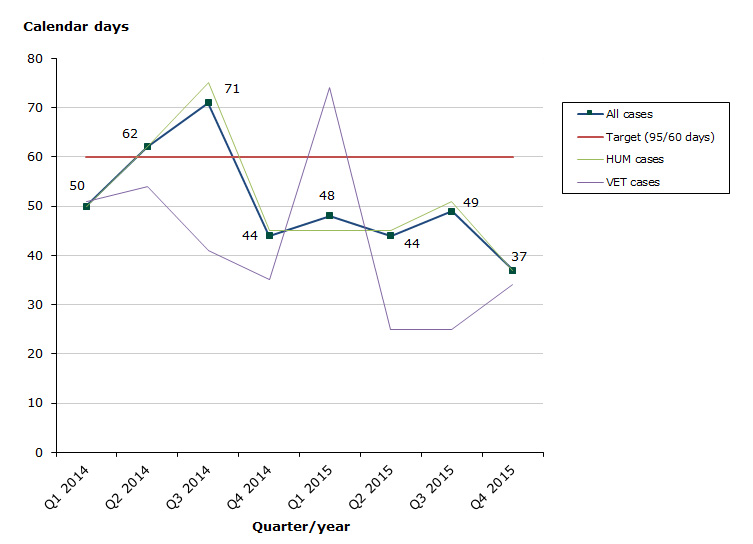
See table 4-6 for type IB variations
Type II variations
Assessment time in 2015 for type II variations as at end-December 2015: 61 % of the cases finished within the performance requirement's maximum of 150 days (171 days on average).
- Human medicines: 61 % of the cases finished within the performance requirement's maximum of 150 days (170 days on average).
- Veterinary medicines: 53 % of the cases finished within the performance requirement's maximum of 150 days (210 days on average).
Chart 3. Assessment times for type II variations in the start-up phase
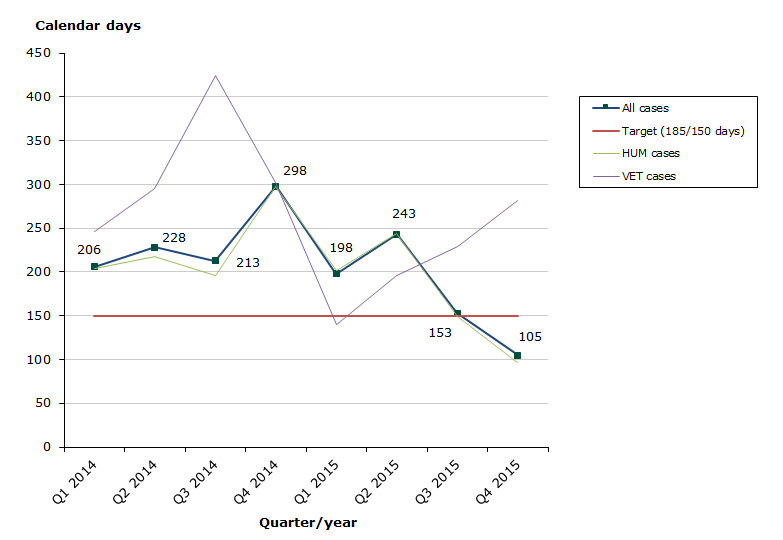
See table 7-9 for type II variations
For further information, please contact Send an email.
