Assessment times for new national applications for the licensing of medicinal products 2015
The assessment times are divided respectively in human and veterinary medicines. The assessment time of new national applications for the licensing of medicinal products, are calculated in the following way:
- Total assessment time
- Assessment time of the start-up phase.
- Assessment time of remaining phases
Initiated cases with a completed start-up phase
- Assessment times of these cases
For Q4 2015, we make the following observations:
In 2015, we have completed 36 cases, of which 23 cases (64 %) where completed within the performance requirement of 240 days. In 2014, 67 % of all cases where finished within the performance requirement, while in 2013 93 % of all cases where finished within the performance requirements.
One of the reasons for the drop of share of cases that are finished within the performance requirement in 2014 and 2015 compared to 2013, is that there has been a 22 % increase in the number of received cases per year in 2014 and 2015, compared to 2013. This increase gives an additional pressure on our resources for the first assessment. Additionally, the amount of completed cases per year in 2014 and 2015, has increased 10% compared to 2013, of which there have been several case backlog than normal.
While focusing on the total assessment time, we make every effort to meet the maximum time allowed in the start-up phase, and Chart 2 below shows the assessment time for cases with a completed start-up phase (i.e. the assessment time is measured from the close of the start-up phase).
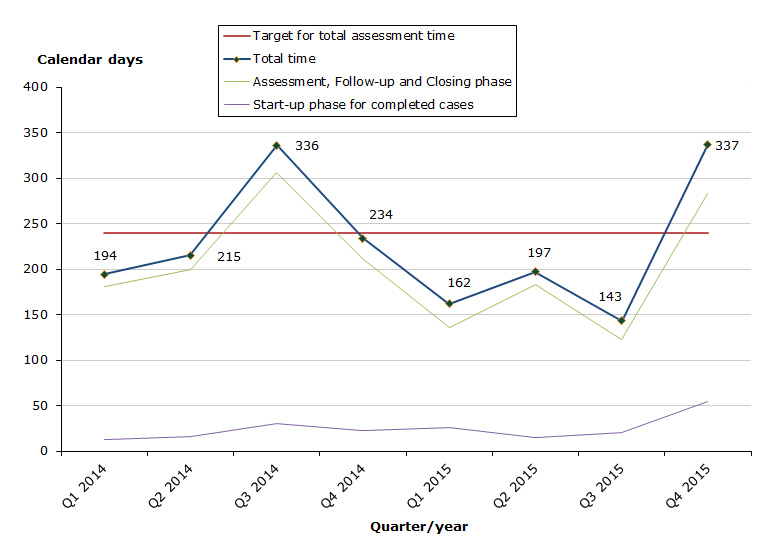
Total assessment times for completed cases
Status for 2015 as at end-December 2015: 36 cases have been completed, 23 cases for human medicines and 13 cases for veterinary medicines.
Total for human and veterinary medicines
- 64 % of the cases finished within the performance requirement's maximum of 240 days (183 days on average).
- 69 % of the cases finished within the performance requirement's maximum of 30 days in the start-up phase (26 days on average).
- 67 % of the cases finished within the performance requirement's maximum of 210 days in the assessment and closing phases (157 days on average).
Human medicines
- 78 % of the cases finished within the performance requirement's maximum of 240 days (144 days on average).
- 65 % of the cases finished within the performance requirement's maximum of 30 days in the start-up phase (22 days on average).
- 74 % of the cases finished within the performance requirement's maximum of 210 days in the assessment and closing phases (122 days on average).
Veterinary medicines
- 38 % of the cases finished within the performance requirement's maximum of 240 days (251 days on average).
- 77 % of the cases finished within the performance requirement's maximum of 30 days in the start-up phase (32 days on average).
- 54 % of the cases finished within the performance requirement's maximum of 210 days in the assessment and closing phases (220 days on average).
Chart 1. Assessment times for completed cases
See table 1-9 for completed cases
The total assessment time is measured against the target time of 240 days for 2015 from the date on which we receive the application until the date of determination (grant or refusal), excluding any clock-stop days.
The assessment time in the start-up phase is measured against the target time of 30 days for 2015 from the date when we receive the application until the procedure start date (day 0).
The assessment time for the remaining phases is measured against the target time of 210 days for 2015 from the procedure start date (day 0) until the date of determination (grant or refusal), excluding any clock-stop days.
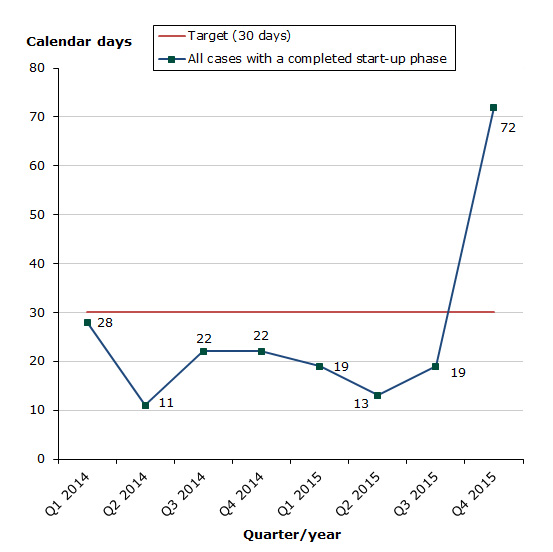
Assessment times for initiated cases
Status for 2015 as at end-December 2015:
- Total for human and veterinary medicines: 68 % of the cases finished within the performance requirement's maximum of 30 days (17 days on average).
- Human medicines: 71 % of the cases finished within the performance requirement's maximum of 30 days (22 days on average).
- Veterinary medicines: 33 % of the cases finished within the performance requirement's maximum of 30 days (40 days on average).
Chart 2. Assessment times for initiated procedures
See table 10-12 for cases with an initiated procedure
The reason for that the aim of 30 days is not met in Q4 2015, is that one application unfortunately was not processed correctly and therefore delayed.
The assessment time is measured against the target time of 30 days for 2015 from the date when we receive the application until the procedure start date (day 0).