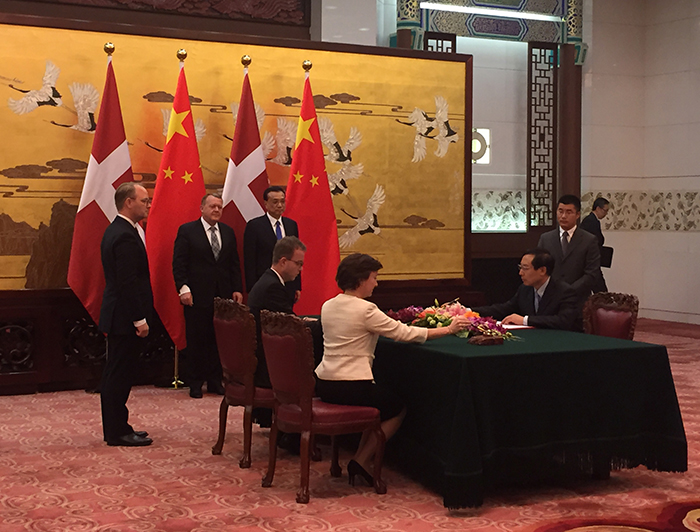
Agreement on Chinese-Danish cooperation signed
The Danish Medicines Agency and the China Food and Drug Administration have taken an important step towards closer cooperation.

Danish Minister for Health Karen Ellemann has signed an agreement that will pave the way for a new Chinese-Danish centre. This agreement is a Memorandum of Understanding, and it provides the framework for the authority-to-authority cooperation between the Danish Medicines Agency and the China Food and Drug Administration.
The signing of the agreement formed part of the programme for the Danish delegation headed by Prime Minister Lars Løkke Rasmussen that is currently visiting China. In addition to the Danish Minister for Health Karen Ellemann and the Danish Minister for Environment and Food Esben Lunde Larsen, Thomas Senderovitz, Director General of the Danish Medicines Agency, and Jakob Cold, Deputy Director General, as well as a large number of Danish business representatives also take part in the visit.
The new China-Denmark Food and Drug Regulatory Cooperation Centre provides the framework for cooperation between the authorities in a wide range of areas, for example medicines licensing, inspections, pharmacovigilance, quality management, laboratory control and clinical trials.
”To the Danish Medicines Agency, this agreement is a very important manifestation of the Chinese-Danish cooperation. It gives us a great opportunity for experience exchange in a wide range of fields. For example in relation to laboratory control as Denmark is the only medicines agency in Europe controlling radiopharmaceuticals. In this field, cooperation and knowledge sharing with the China Food and Drug Administration may prove to be valuable because they are also controlling radiopharmaceuticals. Moreover, this agreement will raise Denmark’s profile as a life science nation internationally,” says Thomas Senderovitz, Director General of the Danish Medicines Agency.
With the formalised cooperation and dialogues with the Danish Medicines Agency, China will get access to know-how about medicines regulation in a European context.
”Of course, we expect that both authorities will benefit from this cooperation. In relation to discussions of specific problems, such as how do the authorities handle 3D printed medical devices? We are very excited to learn from China’s experience. But also in relation to the ongoing development of our internal working procedures. If we describe our procedures to third parties, they may ask questions about things that we take for granted – and that is always a good thing,” says Thomas Senderovitz.