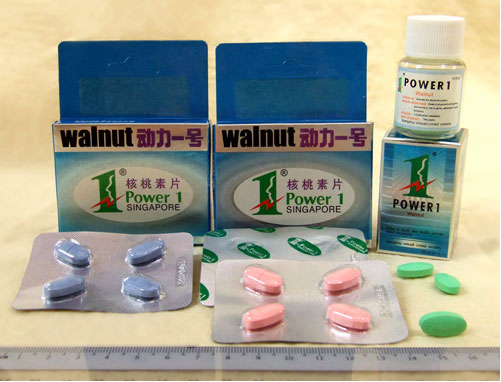
Warning about Power 1 Walnut
The Danish Medicines Agency has been made aware that the Health Sciences Authority in Singapore has confiscated large quantities of the illegal medicinal product Power 1 Walnut, which has been marketed as a potency-enhancing product containing only herbs. An analysis of the product has shown that Power 1 Walnut contains the two active substances sildenafil and glibenclamide.
The presence of sildenafil is both illegal and dangerous, but it is even more alarming that the product contains the active substance glibenclamide, which is used to treat patients with diabetes. In the worst case scenario, glibenclamide may cause death. The Danish Medicines Agency therefore strongly warns against Power 1 Walnut.
Analyses of Power 1 Walnut have shown that it contains overdose of glibenclamide far beyond the normal therapeutic dose. Glibenclamide is included in approved prescription-only medicinal products for the treatment of diabetes. Treatment with glibenclamide should only be commenced on advice from a doctor. Over-concentrations of glibenclamide could lead to abnormally low blood sugar (hypoglycaemia) characterised by symptoms of weakness, cold sweats, hunger, inability to co-ordinate muscle movements (ataxia), double vision, cramps, unconsciousness, permanent brain damage and in the worst case scenario death. The symptoms may develop late - in some cases up to two days after the product has been taken.
The Danish Medicines Agency advises persons who have taken Power 1 Walnut to contact their own doctor regardless of whether or not they have experienced any adverse reactions from the product.
The Danish Medicines Agency has not been informed that Power 1 Walnut is being sold in Denmark, however, since it is likely that the product is sold from foreign websites, it cannot be ruled out that Danes have bought the product on the internet.
The Danish Medicines Agency urges persons who buy medicinal products on the internet to be careful and to seek advice from their doctor before buying prescription-only medicinal products. The doctor may then assess the effect of the product and its adverse reactions, including whether or not the product concerned would benefit the patient.
The press release of the Health Sciences Authority in Singapore and a picture of the product is available in the factbox to the right.