Product defects and withdrawal of medicinal products in 2007
Introduction
Summary of findings in 2007
Reports
- Number of reported product defects
- Reports broken down by source
- Reports broken down by type of defect
- Counterfeit medicinal products
Withdrawals
- Number of withdrawals
- Withdrawals broken down by reporting source
Introduction
The Danish Medicines Agency must be informed through reporting if a company considers a product defect may lead to the withdrawal of a medicinal product from the market. This is stipulated in section 30 of Executive Order no. 1242 of 12 December 2005 on the manufacturing and import of medicinal products and intermediary products. We also receive reports about product defects from foreign medicines agencies via the so-called 'Rapid Alert System'. The reported product defects thus come from both Danish and foreign pharmaceutical companies.
The Danish Medicines Agency carefully considers such reports, which also implies that we assess the gravity of the product defects. Before a decision is made to withdraw a medicinal product from the market, several aspects must be examined, among other things whether the medicinal product is marketed in Denmark, whether it is being clinically tested or dispensed via a special compassionate use permit. Moreover, it is also likely that the medicinal has been exported from Denmark.
Companies must comply with the rules on good manufacturing and distribution practice (GMP and GDP). The rules contribute to minimising potential defects during the manufacturing process and distribution of medicinal products. At inspections, we check whether companies comply with these rules. In addition, we regularly purchase selected medicinal products, packages, labels and analyses for laboratory testing at the Danish Medicines Agency. During case handling of variations of marketing authorisations, information submitted by companies might uncover defects or changes in medicinal products.
Information about medicinal product defects or non-compliance with GMP and GDP therefore also come from the Danish Medicines Agency's own control and licensing activities or from inspection of companies.
If a defective medicinal product is on the Danish market, or if it has been exported from Denmark, we effect a withdrawal in collaboration with the company. It is estimated how far down the chain of distribution the medicinal product is to be withdrawn (wholesaler, pharmacy, consumer), and whether it is relevant to inform other medicines agencies about the withdrawal. Where critical product defects are concerned, we place warnings on our website, www.dkma.dk. Where less critical product defects are concerned, we also consider how a withdrawal of the medicinal product from the market would effect the supply of medicine to the Danish people.
The Danish Medicines Agency may order the withdrawal of a medicinal product, however, withdrawals most often take place in collaboration with the responsible companies.
Summary of findings in 2007
In 2007, 202 reports about medicinal product defects were registered, which is basically on level with 2006. 28% of these reports resulted in a withdrawal from the Danish market, which is exactly the same proportion as the year before.
In 2007, the Danish Medicines Agency received 19 warnings about counterfeit medicinal products in the legitimate global supply chain, which is more than a two-fold increase on 2006. Still, there have been no reports of counterfeit medicinal products in the legitimate supply chain in Denmark.
Reports
Number of reported product defects
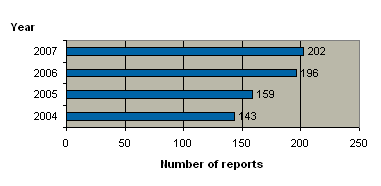
In 2007, we received a total of 202 reports on medicinal product defects. Figure 1 shows the number of reports received in the period 2004-2007. In 2007, the number of reports increased only marginally on 2006, which, after all, was a year that had seen a drastic increase on the year before.
Figure 1. Number of reports on product defects 2004-2007
The 202 reports about product defects in 2007 both concern marketed and non-marketed medicinal products, and cover
- Reports about product defects from companies and authorities (> 90% of the reports)
- Complaints from medicine users
- Discovery of counterfeit medicinal products which are not manufactured by an authorised pharmaceutical manufacturer, and where quality, effect and safety are not documented
- Reports about non-compliance with GMP or GDP at companies, uncovered from inspections carried out by European medicines agencies.
Figure 2 shows the 202 reports received in 2007 broken down by source.
Figure 2. Reports broken down by source
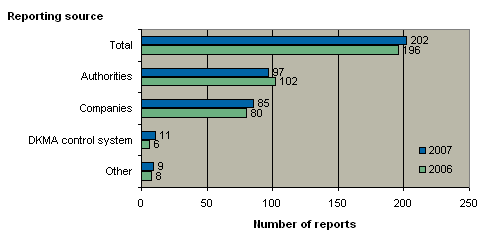
In 2007, more reports came from the Danish Medicines Agency's own control system than was the case in 2006. Likewise, the year recorded a small increase in the number of reports submitted by companies (manufacturers and importers) and a small decline in the number of reports from other medicines agencies compared with 2006. The number of reports from other sources (patients, physicians and pharmacies) was largely on level with 2006.
Reports broken down by type of defect
When reports are registered at the Danish Medicines Agency, they are divided into six different types of defect as illustrated by figure 3 below. Box 1 briefly describes each of the six types of defects.
Figure 3. Reports broken down by type of defect
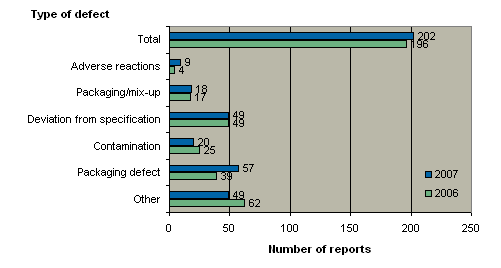
Compared with 2006, there were more reports of adverse drug reactions and packaging defects in 2007. By contrast, the number of reported defects related to contamination and 'other deviations' fell on 2006. By and large, the figures for defects related to packaging/mix-up and deviation from specifications remained unchanged in 2007.
Box. 1. Types of defect
|
Counterfeit medicinal products
The Rapid Alert System reported an increasing number of warnings about counterfeit medicinal products in the legitimate supply chain in the period 2003-2007, cf. table 1. This is evidence that an increasing number of counterfeit medicine apparently enters the global market. Despite this trend, the Danish Medicines Agency has not received any reports about counterfeit medicinal products in the legitimate supply chain in Denmark so far.
Table 1. Number of reports about counterfeit medicinal products in the period 2003-2007.
2003 |
2004 |
2005 |
2006 |
2007 | |
Counterfeit medicinal products |
0 |
2 |
3 |
8 |
19 |
As part of our activities to prevent counterfeit medicinal products from reaching consumers, we have taken the initiative to form a national network against counterfeit medicinal products. The network is made up of authorities, industrial associations and all links in the supply chain. The network participants meet regularly to discuss issues related to counterfeit medicinal products to initiate local and cross-cutting precautionary measures.
Withdrawals
Of the total of 202 reports on product defects that we received in 2007, 28% resulted in withdrawals of medicinal products from the Danish market.
Consequently, the proportion of withdrawn products is unchanged compared with 2006, cf. table 2 below, and reflects a declining trend compared to the previous years, 2004-2005.
Table 2. Number of withdrawals in the period 2002-2007.
2002 |
2003 |
2004 |
2005 |
2006 |
2007 | |
Number of reports |
192 |
150 |
143 |
160 |
196 |
202 |
Number of withdrawals |
72 |
39 |
58 |
58 |
55 |
57 |
Withdrawals in per cent |
38 % |
26 % |
41 % |
36 % |
28 % |
28 % |
Withdrawals broken down by reporting source
Table 3 shows how the 57 withdrawals in 2007 are distributed on reporting source.
Table 3. Reports resulting in withdrawals broken down by source in 2006-2007
Number in 2006 |
Number in 2007 | |
Company |
39 |
47 |
Foreign authority |
10 |
4 |
Danish Medicines Agency's control system |
2 |
5 |
Other |
4 |
1 |
Total |
55 |
57 |
The table illustrates that the number of withdrawals caused by reports from companies increased compared with 2006. This is also the case for withdrawals resulting from product defects notified via the Danish Medicines Agency's control system. By contrast, 2007 saw a decline in withdrawals occasioned by product defects reported from foreign authorities and 'others' (patients, physicians, pharmacies) compared to 2006.
The 47 withdrawals that were based on reports from companies were primarily caused by packaging defects. The product defect 'specification deviations' and 'other deviations' also accounted for a good share of these withdrawals.
Danish Medicines Agency, 19 May 2008.