Annual report of the Reimbursement Committee 2009
Members appointed by the Danish Minister for the Interior and Health 17 meetings in 200921 applications for general reimbursement Grounds1632 applications for individual reimbursement Annual review of the number of applications for single reimbursementMeeting of the Reimbursement Committee's group of experts Meeting between the Nordic authoritiesThe Institute for Rational Pharmacotherapy's autumn conference for regional drug treatment advisers Reassessment of the reimbursement status of medicinal products
The Reimbursement Committee advises the Danish Medicines Agency on applications regarding reimbursement for medicines. This goes for both general reimbursement (applied for by pharmaceutical companies) and reimbursement for individual patients (applied for by a physician). Furthermore, the Committee advises the Danish Medicines Agency when the Agency reviews the reimbursement status of medicinal products. The Danish Medicines Agency acts as secretariat to the Committee.
Members appointed by the Danish Minister for the Interior and Health
The Committee has up to seven members of which two must be general practitioners. The members are appointed by the Danish Minister for the Interior and Health upon nomination by the Danish Medicines Agency. One member is appointed by the Regions' Board for Wages and Tariffs. The Committee members are appointed for four years, and together they have a wide-ranging expertise. The members' declaration of interest forms can be seen at www.dkma.dk in Danish.
In 2009, the members of the Reimbursement Committee were:
MD Mogens Laue Friis (Chairman),
MD Karine Bech,
GP Ellen-Christine Beiter,
GP Palle Mark Christensen, from 1 July,
MD Thomas Gjørup, from 1 July,
Director of the Centre of Diagnostic Investigations, MD, Mogens Sandbjerg Hansen, until 1 July,
GP John Larsen,
Head of Development, Peder Ørnsholt Ring (Danish Regions).
1 July 2009 marked the beginning of a new four-year term of the Reimbursement Committee. While Director of the Centre of Diagnostic Investigations, MD, Mogens Sandbjerg Hansen left the Committee, two new members joined: GP Palle Mark Christensen upon nomination by the Danish Society of Clinical Pharmacology and MD Thomas Gjørup upon nomination by the Danish Society of Internal Medicine.
17 meetings in 2009
In 2009, the Reimbursement Committee held 17 meetings of which six addressed the review of the reimbursement status of medicines. The minutes are available at www.dkma.dk.
21 applications for general reimbursement
The Reimbursement Committee gives advice to the Danish Medicines Agency on general reimbursement applications pursuant to the criteria laid down in Danish executive order no. 180 of 17 March 2005 on reimbursement.
In 2009, the Reimbursement Committee reviewed 21 general reimbursement applications, many of which were transacted at more than one meeting. Nine medicines were recommended for general unconditional reimbursement, two were recommended for general conditional reimbursement, one of which was recommended for extension. 10 medicines were recommended for refusal, one of which resulted in the change of reimbursement status from general unconditional reimbursement to general conditional reimbursement. Four of the applications were reapplications. Two of the applications were postponed for consideration at subsequent meetings. One application because the Committee needed a statement from a scientific society before it could reach a final decision on the matter. The other because the Committee wanted the Danish Institute for Health Services Research to assess a health economic analysis submitted by the company concerned. The number of applications for general reimbursement reviewed by the Committee is shown in figure 1.
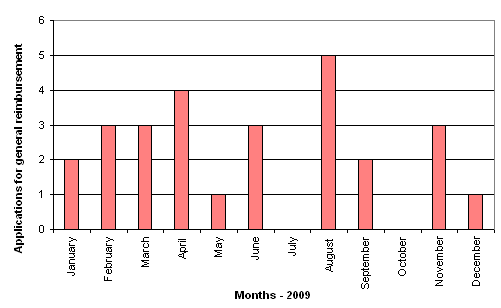
Figure 1. Number of applications for general reimbursement reviewed by the Committee per monthly meeting in 2009.
Grounds
The Committee's recommendations regarding general reimbursement applications are published on the Danish Medicines Agency's website. The recommended refusals in 2009 were justified in e.g. the price of the concerned medicine compared to other comparable products and the risk that the concerned medicine would be used as first-line treatment even though the Committee found this to be inappropriate.
Most often, the grounds for recommending general reimbursement were that the therapeutic value was found to be commensurate with the price of other comparable medicines.
1632 applications for individual reimbursement
In 2009, the Danish Medicines Agency reached decisions in a total of 138,715 individual reimbursement applications. 1632 of these applications were presented to the Reimbursement Committee because the Secretariat found that individual reimbursement could not be granted offhand. In 31 % of these applications, the Committee recommended that individual reimbursement should be granted. The number of applications for individual reimbursement reviewed by the Committee is shown in figure 2.
When the Reimbursement Committee reviews single reimbursement applications, it regularly discusses if the guiding criteria for single reimbursement of the many medicines still comply with the most recent knowledge within the disease area concerned. These discussions led to the updating of the guiding criteria for single reimbursement of medicines for neuropathic pain, grass pollen allergy and refractory, severe hand eczema during the course of 2009.
Annual review of the number of applications for single reimbursement
Every year, the Danish Medicines Agency makes a report on the number of single reimbursement applications submitted over the year. Based on this report, the Committee discusses whether the distribution of granted and refused applications for the respective medicines/medicine groups, and the volume of applications, is desirable. In 2009, no medicines/medicine groups were recommended for general or general conditional reimbursement as a result of these discussions.
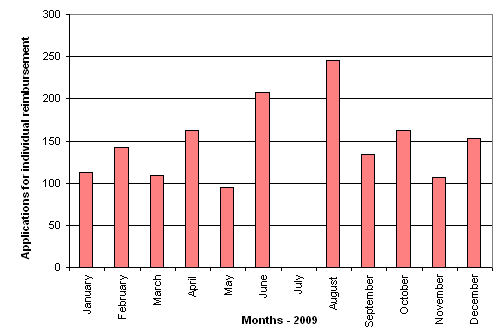
Figure 2. Number of applications for individual reimbursement reviewed by the Committee per monthly meeting held in 2009.
Meeting of the Reimbursement Committee's group of experts
The Reimbursement Committee's group of experts, which had been composed to discuss the question of reimbursement for alitretinoin, held a meeting on 12 May 2009. The group of experts consists of representatives from the Danish Society of Dermatology and two members of the Reimbursement Committee - Chairman Mogens Laue Friis and Ellen-Christine Beiter. The Danish Medicines Agency served as secretariat.
The group of experts convened to discuss and possibly review the current guiding criteria for single reimbursement of alitretinoin for the treatment of patients with refractory, severe hand eczema.
Meeting between the Nordic authorities
Mogens Laue Friis participated in a meeting between the Nordic authorities held in Oslo from 10 to 11 September. The purpose of the meeting was primarily to discuss topics of special interest to the price and reimbursement authorities in the Nordic region and to be updated on developments having occurred in the individual countries since the last meeting. Sweden presented a large-scale restructuring of the pharmacies, including discontinuation of the pharmacy monopoly. Finland presented a new reference price system, and a proposal for giving nurses the right to prescribe medicine. Iceland presented different initiatives launched to lower pharmaceutical costs. Norway presented a new price survey, experience with a new indication-based reimbursement list and improvements of the single reimbursement scheme inspired by a field trip to Denmark. Denmark presented the newly changed reimbursement status for cardiovascular products, a report on hospital medicines and an agreement on price ceilings. Finally, a number of new medicinal products were discussed from the perspective of reimbursement status in the individual countries.
The Institute for Rational Pharmacotherapy's autumn conference for regional drug treatment advisers
On 6 October 2009, Mogens Laue Friis spoke about reimbursement for medical treatment of ADHD at the Institute for Rational Pharmacotherapy's autumn meeting for regional drug treatment advisers in Denmark. Every year, the Institute for Rational Pharmacotherapy arranges a conference for the regional drug treatment advisers with the purpose of exchanging experience and viewpoints to enhance and strengthen the efforts of regional drug treatment advisers.
Reassessment of the reimbursement status of medicinal products
Pursuant to the Danish Health Act, the Danish Medicines Agency must regularly review the reimbursement status of all medicines. The Reimbursement Committee prepares recommendations for the future reimbursement status of medicines for the Danish Medicines Agency. The key criteria for determining the priority in which the individual groups of medicines should be reassessed are: the significance of the medicines to the primary sector and especially general practice, public health aspects, new evidence-based recommendations, large expenses for patient and region as well as a large consumption. Based on these criteria, the Reimbursement Committee recommended in 2009 that the Danish Medicines Agency in 2010 begin reassessing the reimbursement status of the following groups of medicines, in the order mentioned: Antibacterials for systemic use, antidepressants, opioids and antidiabetics.
In 2009, the Committee discussed the reimbursement status of the following medicine groups:
- Laxatives,
- Antiobesity preparations, excl. diet products,
- Antiemetics and antinauseants,
- Propulsives,
- Peripheral vasodilators,
- Vasoprotectives,
- Drugs for acid-related disorders.
In addition, the Committee has kept a close watch on blood pressure medicines, including changes in consumption patterns before and after the reimbursement change of 13 July 2009.
Out of the medicine groups listed above, the Committee recommendations for antiobesity preparations, excl. diet products, laxatives and magnesium hydroxide, antiemetics and antinauseants, propulsives, peripheral vasodilators, vasoprotectives and drugs for acid-related disorders were submitted for consultation among the affected companies, relevant organisations and scientific societies. The consultation responses are available in Danish at www.dkma.dk.
The consultation deadline for acid-related disorders passed on 15 March 2010. Only one other medicine group had generated consultation responses. The replies were discussed by the Committee, which did not find reason to change its recommendation. The Danish Medicines Agency followed the Committee's recommendations and has reached decisions for the following groups: laxatives, antiobesity preparations, excl. diet products, antiemetics and antinauseants, propulsives and peripheral vasodilators. Changes of reimbursement status were effected for metoclopramide (A03FA01) tablets and hard prolonged-release capsules and domperidone (A03FA03), which became eligible for general reimbursement on 14 December 2009. The reimbursement status of the remaining medicinal products remained unchanged. The decisions of the Danish Medicines Agency can be read at www.dkma.dk.
Reimbursement Committee
Danish Medicines Agency, 23 April 2010
