Product defects and withdrawal of medicinal products in 2009
Summary of findings in 2009
1. Reports
1.1 Number of reported product defects
1.2 Reports broken down by source
1.3 Reports broken down by type of defect
1.4 Counterfeit medicines
2. Withdrawals
2.1 Number of withdrawals
2.2 Withdrawals broken down by reporting source
The Danish Medicines Agency must be informed through reporting if a company considers a product defect may lead to the withdrawal of a medicinal product from the market. This is stipulated in section 30 of executive order no. 1242 of 12 December 2005 on the manufacturing and import of medicinal products and intermediary products.
Via the so-called ‘Rapid Alert System’, the Danish Medicines Agency receives warnings about product defects from foreign medicines agencies and reports new product defects at international level. The Rapid Alert System covers medicinal products in the legal supply chain only.
The reports are divided into three classes: I, II and III according to the severity of the defect. Class I defects are potentially life threatening and require the dispatch of rapid alerts to all parties. Class II and class III defects could involve patient risk and are treated accordingly. The Danish Medicines Agency’s reports for 2009 mainly constitute class I and class II defects. Danish and foreign companies can also report product defects to the Danish Medicines Agency directly.
The Danish Medicines Agency investigates all reports thoroughly. Before we decide to withdraw a medicinal product from the market, several aspects must be examined, among other things whether the medicinal product is marketed in Denmark, whether it is being clinically tested or dispensed via a special compassionate use permit. In addition, the extent to which the product defect presents a potential risk for the patient is investigated. Moreover, the medicinal product might have been exported from Denmark. The reports cover human as well as veterinary products.
If a defective medicinal product is on the Danish market, or if it has been exported, the Danish Medicines Agency may effect a withdrawal in collaboration with the company. We estimate how far down the supply chain the medicinal product needs to be withdrawn (wholesaler, pharmacy, consumer), and whether it is relevant to inform other medicines agencies about the withdrawal. Where critical product defects are concerned, we place warnings on our website, www.dkma.dk. Where less critical product defects are concerned, we also consider how a withdrawal of the medicinal product from the market would effect the supply of medicine to the Danish people.
Companies must comply with the rules on good manufacturing and distribution practice (GMP and GDP). The rules contribute to minimising potential defects during the manufacturing process and distribution of medicinal products. At inspections, we check whether companies comply with these rules. In addition, we regularly take in selected medicinal products, packages, labels, package leaflets and analyses for testing in our laboratories. During the review of variations of marketing authorisations, submitted information might uncover defects or changes in medicinal products. Therefore, the withdrawal of medicinal products could also be effected in response to the Danish Medicines Agency’s own control activities and authorisation of medicinal products or as a result of company inspections.
Summary of findings in 2009
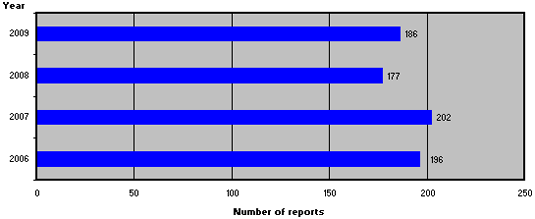
In 2009, 186 reports of medicinal product defects were registered, which is slightly more than in 2008. Compared to 2008, the reporting sources and types of defects have shifted, and reports from the Danish Medicines Agency's own control system accounted for a larger share in 2009 compared to 2008. Six of the 186 reports were caused by counterfeit medicines, which, however, did not affect the Danish market. Approx. 25 % of the reports resulted in withdrawals of medicinal products from the Danish market. The majority of the withdrawals were reported by the companies and were primarily caused by 'deviations from specifications', 'packaging defects' as well as 'packaging, bottling/filling and labelling'.
1. Reports
1.1 Number of reported product defects
In 2009, 186 reports of medicinal product defects were registered, which is a relatively small increase compared to 2008.
Figure 1 shows the number of reports from 2006-2009.
Figure 1. Number of product defect reports from 2006-2009
The 186 product defect reports in 2009 both concern marketed and non-marketed medicinal products. Only medicinal products are included in the total number of reports, which means that vaccines, food supplements, etc. are not included. The reports cover:
- Product defects from companies and authorities, including deviations from specifications and authorised variation applications (> 87 % of the reports).
- Complaints from medicine users or pharmacies.
- Reports of companies’ non-compliance with GMP or GDP, uncovered from company inspections carried out by the Danish Medicines Agency or another European medicines agency.
- Reports of adverse reactions caused by product defects.
1.2 Reports broken down by source
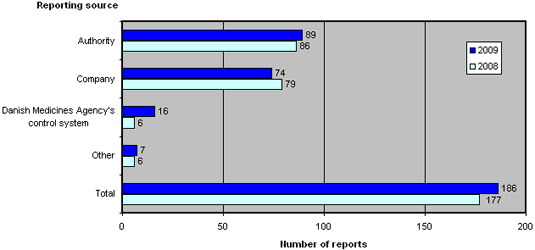
Figure 2 shows the reporting sources of the 186 reports submitted to the Danish Medicines Agency in 2009 and 2008.
Figure 2.Reports broken down by source
Compared to 2008, the reporting sources have shifted. 2009 saw an increase in the number of reports from the Danish Medicines Agency's own control and licensing systems, reports from other medicines agencies as well as other sources, which includes patients, doctors and pharmacies. Conversely, 2009 saw a reduction in cases reported by companies, which includes manufacturers and importers.
In the past couple of years, an increasing number of manufacturers of active pharmaceutical ingredients (APIs) have been reported due to non-compliance with GMP in connection with the manufacturing of APIs.
1.3 Reports broken down by type of defect
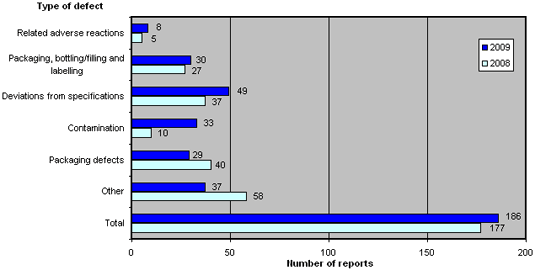
When reports are registered at the Danish Medicines Agency, they are divided into six different types of defects.
The distribution for 2009 compared to 2008 is shown in figure 3. For a brief description of the types of defects, please see Box 1 below.
Figure 3. Reports broken down by types of defect
2009 recorded an increase in the number of defects categorised as 'related adverse reactions', 'packaging, bottling/filling and labelling', 'deviations from specifications' and 'contamination', of which the latter increased significantly. In contrast, the categories 'packaging defects' and 'other' fell compared to 2008.
|
Box 1. Types of defects
|
In the period 2004-2007, the Rapid Alert System transmitted an increasing number of warnings about counterfeit medicines found in the legitimate supply chain globally. After a considerable decline in counterfeit medicines in 2008, we once again recorded a small increase in 2009, see table 1. The increase could be a result of intensified focus on counterfeits coupled with a rise in the number of reports submitted to the Danish Medicines Agency.
We have not received information about or observed any counterfeit medicines in the legitimate supply chain in Denmark so far.
Table 1. Number of reports of counterfeit medicines in the period 2004-2009.
|
|
2004 |
2005 |
2006 |
2007 |
2008 |
2009 |
|
Counterfeit medicines |
2 |
3 |
8 |
19 |
3 |
6 |
As part of our activities to prevent counterfeit medicines from reaching consumers, the Danish Medicines Agency has set up a network consisting of authorities, industrial associations, scientific organisations and all links in the supply chain. The network participants meet biannually and initiate local and cross-cutting preventive measures. The network has produced guidelines for pharmaceutical companies and pharmacies in efforts to prevent counterfeit medicines from entering the legal supply chain.
In connection with the Danish Medicines Agency's work in the interdepartmental 'Network against Copyright Piracy', structured dialogue has been opened with the industry on the combating of counterfeit products in general, which also includes counterfeit medicines.
In order to facilitate the reporting procedure for companies comprised by the reporting duty of section 43B of the Danish Medicines Act (act no. 1180 of 12 December 2005), we have placed an electronic reporting form on our website (in Danish only). The reporting duty was implemented by amendment of the Danish Medicines Act on 1 July 2008 and obligates marketing authorisation holders pursuant to section 7 and holders of a company authorisation pursuant to section 39(1) to report any discovery of counterfeit medicines to the Danish Medicines Agency. In 2009, we received one report on the discovery of a counterfeit medicine in the illegal supply chain in Denmark, and we know of one more case concerning discovery of counterfeit medicines in the illegal supply chain in Denmark. We are presently developing guidelines on the companies' reporting duty.
The Danish Medicines Agency participates in the European cooperation between medicines agencies in the EU, which exchanges information about illegal, including counterfeit, medicinal products distributed in the illegal supply chain in Europe. This could concern pharmaceuticals sold illegally via the internet, which could be related to a multitude of countries. We investigate such information if it seems to be related to Denmark and sanctioning measures are required. In circumstances that may be penalised under the Danish Medicines Act, the Danish Medicines Agency makes decisions regarding the liable party and refers, according to the circumstances, cases to the police for imposition of legal penalties. In addition, we publish warnings on our website whenever it is necessary to inform Danish consumers.
In 2009, the Danish Medicines Agency also participated in a joint global operation (Operation Pangea II) against online distribution of illegal, including counterfeit, medicinal products. 25 countries participated in the operation to put joint focus on this transnational problem.
25% of the 186 product defects reported to the Danish Medicines Agency in 2009 resulted in actual withdrawals of medicinal products from the Danish market.
Figure 2 shows that the total number of reports increased compared to 2008. We also recorded an increase in the number of withdrawals, after a period from 2004-2008 when withdrawals were on a downward trend.
Table 2. Number of withdrawals from 2004-2009
|
|
2004 |
2005 |
2006 |
2007 |
2008 |
2009 |
|
Reports |
143 |
160 |
196 |
202 |
177 |
186 |
|
Number of withdrawals |
58 |
58 |
55 |
57 |
41 |
46 |
|
Withdrawals in per cent |
41% |
36% |
28% |
28% |
23% |
25% |
2.2 Withdrawals broken down by reporting source
Table 3 breaks down the 46 withdrawals in 2009 on reporting source.
Table 3. Reports resulting in withdrawals broken down by reporting source in 2007-2009
|
|
Number in 2007 |
Number in 2008 |
Number in 2009 |
|
Company |
47 |
29 |
29 |
|
Foreign authority |
4 |
8 |
5 |
|
Danish Medicines Agency’s control system |
5 |
3 |
8 |
|
Other |
1 |
1 |
4 |
|
Total |
57 |
41 |
46 |
The distribution of withdrawals in 2009 only shifted slightly compared to 2008. While the number of withdrawals caused by reports from companies is unchanged compared to 2008, there has been an increase in the number of medicinal products withdrawn on the basis of reports from the Danish Medicines Agency's own control system and other sources (patients, doctors, pharmacies).
The 46 withdrawals in 2009 were primarily caused by 'deviations from specifications', 'packaging defects' and ‘packaging, bottling/filling and labelling’.
For further information, please contact Charlotte Henriksen, tel. +45 44 88 92 51.
Danish Medicines Agency, 15 June 2010