Clinical trials with medicinal products
Did you know that Denmark is the country in the EU conducting the most clinical trials per million inhabitants?
The new ambitious life science strategy (In Danish only) from November 2024 focuses on keeping Denmark at the forefront - and this is now resulting in an extraordinary effort for early-phase research in collaboration with the Medical Research Ethics Committees (MREC).
14-day assessment time for phase I trials
Find the information on expedited assessment compiled HERE.
The process will initially apply automatically to all mono-national phase I and phase I-II trials. At the same time, we will strive to accommodate rapid assessment of multinational trials by further agreement and coordination.
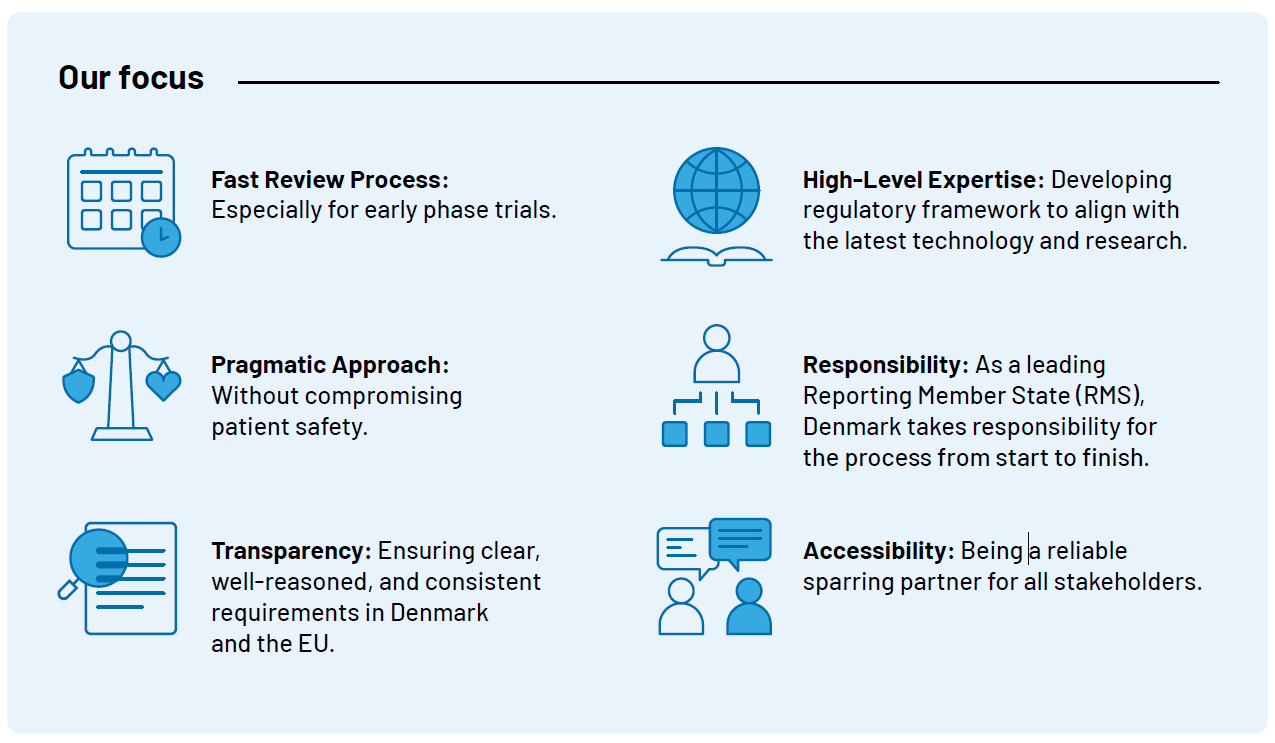
Key Focus Areas in 2025
- Processing Times for Clinical Trials
For mononational trials in Denmark we prioritize swift processing times. The goal is to provide responses for mononational trials within 31 days. Additionally, efforts are underway to ensure even faster processing times for early phase trials.
- Combined Studies with Medicinal Products and Medical Devices
We have developed a national pilot program allowing applications for combined studies with medicinal products and medical devices to be submitted and jointly assessed. Insights from this initiative are being applied in the EU COMBINE project to develop common solutions for this interface within the EU.
- Use of Data, Including Registry Data, and Advanced Data Analyses in Clinical Trials
In 2025, we will strengthen our focus on the concept of personalized medicine by enhancing regulatory frameworks and skills for using data and advanced data analyses, including AI/ML, in clinical trials with medicinal products.
- Clinical Trials with Innovative Medicines, Including Advanced Therapies (ATMP)
ATMP is a priority area for clinical trials and the Danish Medicines Agency. We have consolidated information specific to regulatory guidance on ATMP on our website.
The Value of Clinical Trials
Conducting well-designed and safe clinical trials is crucial for gaining knowledge about medicines and is the central part of the foundation for the approval of new, effective and safe medicines. Additionally, clinical trials play a vital role in creating an attractive and competent environment for medicines development and research in Denmark.
Clinical trials provide early insights into new medicinal treatments in the healthcare system, which builds competence - and most importantly, benefits patients by giving them early access to the latest medicines.
At the Danish Medicines Agency, we oversee the entire process of medicinal product development and believe that Denmark has the prerequisites to excel particularly in the development of new clinical trial designs, trials with complex medicines related to rare diseases or personalized medicines - as well as early phase research, including ‘First in Human’ (FIH) trials. This is partly due to Denmark’s skilled and specialized researchers and Denmark’s many technological opportunities.
You can read more about what a clinical trial is on our page: What is a clinical trial?
General Focus Areas
Our Clinical Trials Unit works with the goal to improve the framework for clinical trials in Denmark and the EU in collaboration with stakeholders from the entire ecosystem, always focusing on the best interests of patients. We have close collaboration with the Danish National Center for Ethics, including the Medical Research Ethics Committees (MREC).
Our focus remains on being at the forefront as the leading reporting member state (RMS) on multinational trials, delivering timely high-quality case assessment, while developing guidelines that support of clinical trials of the future. Our work is carried out in ongoing dialogue with our environment and with a pragmatic approach that primarily focuses on the safety and interests of trial participants.
See here for a higher resolution of the illustration.
More information about applying for clinical trials can be found here.
You can read about Medical Research Ethics Committees role on their website.
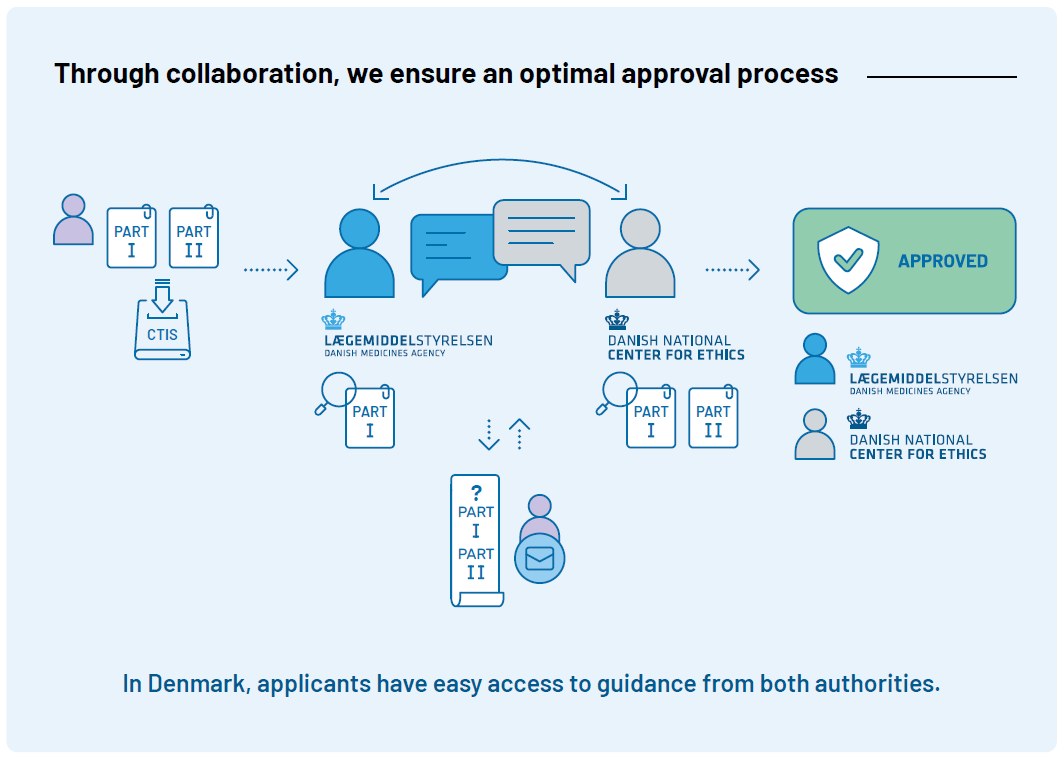
Collaboration on the Approval of Clinical Trials
Clinical trials are approved by both the Danish Medicines Agency and the Medical Research Ethics Committees (MREC), and a joint application with Part I and Part II is submitted via the European data portal; Clinical Trial Information System (CTIS) to both authorities.
We continuously optimize the approval procedure to offer standardized assessments of high quality every time, regardless of the type of trial application. We communicate continuously about application requirements and our processing times to create predictability about the process.
For both mononational and multinational trials we prepare a joint consolidated list of questions for the sponsor for Part I together with MREC. We strive to raise clear, well-founded considerations that are critical for approval. The sponsor can easily identify which questions are raised from DKMA and MREC, respectively. Likewise, we prioritize a quick assessment of the sponsor’s responses so that a second round of questions can be posed to the sponsor if needed: If we estimate for Part I that the trial has more benefits than disadvantages (positive benefit-risk balance), minor deficiencies do not prevent the trial from starting in Denmark.
See here for a higher resolution of the illustration of the approval process.
We prioritize close dialogue with the sponsor, and you can always get in contact with us both by mail and phone. We provide guidance on regulatory requirements or specific scientific issues in clinical trials, both before submitting an application in CTIS, during the assessment of the application, and when the trial is ongoing or being completed.
International Engagement and Focus on Early-Phase Research
We engage nationally and internationally with our stakeholders to foster an innovative environment that benefits patients and research, emphasizing a high professional standard based on the latest knowledge in the field.
In Denmark, we prioritize early phase research. Early phase trials provide Danish patients with access to the newest treatment options and build critical scientific capacity for both us as a regulatory authority and Danish researchers. This is expected to have a positive impact on later stages of medicines development.
We continually implement initiatives to support early research. We possess strong competencies to facilitate the transition from toxicological studies to clinical trials and offer scientific advice on toxicological, clinical, and regulatory matters.
Furthermore, efforts are ongoing to re-establish short processing times for early-phase trials under the regulation.
Danish Expertise Makes a Difference in Europe
Harmonization is a key priority for us, and we dedicate significant resources to aligning the handling and assessment of clinical trial applications within the EU.
We collaborate closely with our European colleagues on this and actively contribute to working groups under, among others, the European Commission, the European Medicines Agency (EMA), and the Heads of Medicines Agencies (HMA). In this context, the Danish Medicines Agency has been responsible for developing EU guidelines on among others complex clinical trials, GLP requirements for non-clinical research and decentralized clinical trials.