Assessment times for issuance of marketing authorisations in Concerned Member State cases 2020
Under the mutual recognition procedure and the decentralised procedure, we have 30 days to issue a marketing authorisation from the date when we receive an acceptable Danish summary of product characteristics (SmPC).
Status in 2020 as at end-December 2020: 80 % of the cases finished within the performance requirement's maximum of 30 days (27 days on average):
- Human medicines: 83 % of the cases finished within the performance requirement's maximum of 30 days (26 days on average).
- Veterinary medicines: 70 % of the cases finished within the performance requirement's maximum of 30 days (30 days on average).
Due to the Covid-19 pandemic, the Danish Medicines Agency has not prioritized the assessments times in 2020. Only in 2022 is it expected that the assessments times will reach the approx. 95% compliance.
Chart 1: Average assessment times for issuance of marketing authorisations with Denmark as Concerned Member State (CMS)
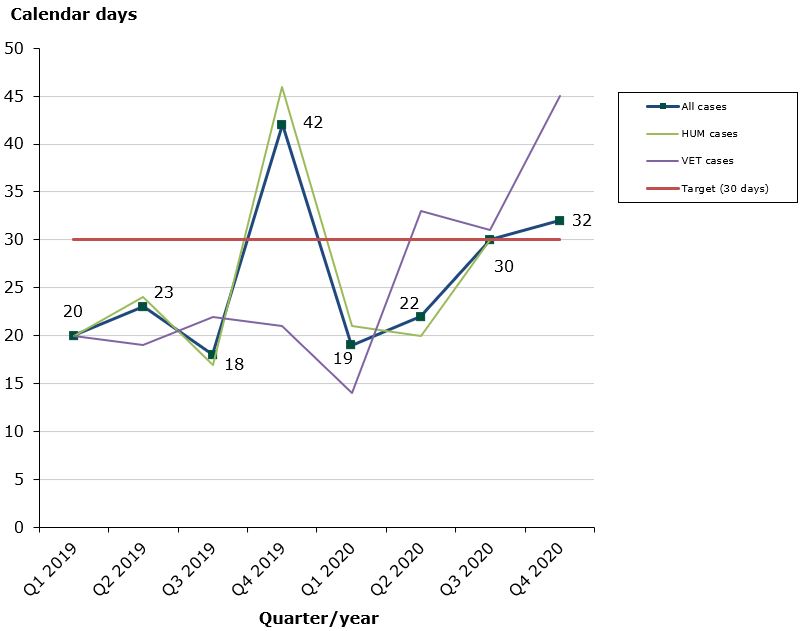
The Danish Medicines Agency has established a best practice agreement on the issuance of marketing authorisations in connection with MRP/DCP applications where Denmark is Concerned Member State. According to this agreement, the maximum assessment time allowed is 30 days from the date on which we receive an acceptable Danish version of the authorised SPC until issuance of a marketing authorisation.
