Investigations of the AstraZeneca vaccine (Vaxzevria)
In this theme, you can read about the drug regulatory authorities’ investigation of the rare and unusual symptoms of low levels of blood platelets, blood clots and bleeding after vaccination with the AstraZeneca COVID-19 vaccine (Vaxzevria).
ThemeThe AstraZeneca COVID-19 vaccine Vaxzevria is one of the four vaccines against COVID-19 so far approved by the European Medicines Agency (EMA).
On 11 March, the EMA started reviewing the rare but serious side effects observed after vaccinations with COVID-19 Vaccine AstraZeneca. A number of investigations have been conducted in parallel in Denmark.
Vaxzevria is probably the cause of rare, serious symptoms with blood clots combined with a low platelet count and bleeding. This has been concluded by an expert group set up by the EMA.
However, the conclusion does not change the status of the AstraZeneca COVID-19 vaccine, which remains a vaccine authorised by the drug regulatory authorities because its benefits of preventing death and other health-related COVID-19 consequences still outweigh the possible risks in the form of known and possible side effects.
Status in Denmark
The Danish Medicines Agency has received two Danish reports confirmed to involve the unusual symptoms, after vaccination with Vaxzevria.
The two Danish cases and the similar cases reported in other countries mainly occurred within 14 days of vaccination. One of the cases in Denmark had a fatal outcome.
On the basis of the link between the AstraZeneca vaccine and these cases with the unusual symptoms and coupled with the fact that the COVID-19 epidemic is generally under control in Denmark and there are other COVID-19 vaccines available, the Danish Health Authority has decided to continue the COVID-19 vaccine rollout without the vaccine from AstraZeneca.
Link to the press release from the Danish Health Authority: Denmark continues its vaccine rollout without the COVID-19 vaccine from AstraZeneca
News from the Danish Medicines Agency
Questions and answers on the AstraZeneca vaccine
What should I do if I have had the COVID-19 vaccine Vaxzevria?
It is important to keep in mind that the reported cases of blood clots accompanied by low levels of blood platelets and bleeding are very rare, and that the vaccines protect against COVID-19 and generally reduce the severity of disease should you develop symptoms even if you have been vaccinated. You should therefore not be worried if you have received the vaccine, but you should pay attention to symptoms for 14 days after vaccination. Read the Danish Medicines Agency’s letter of 15 March 2021 sent out to citizens having received the AstraZeneca vaccine.
What are the most common side effects seen after vaccination with Vaxevria?
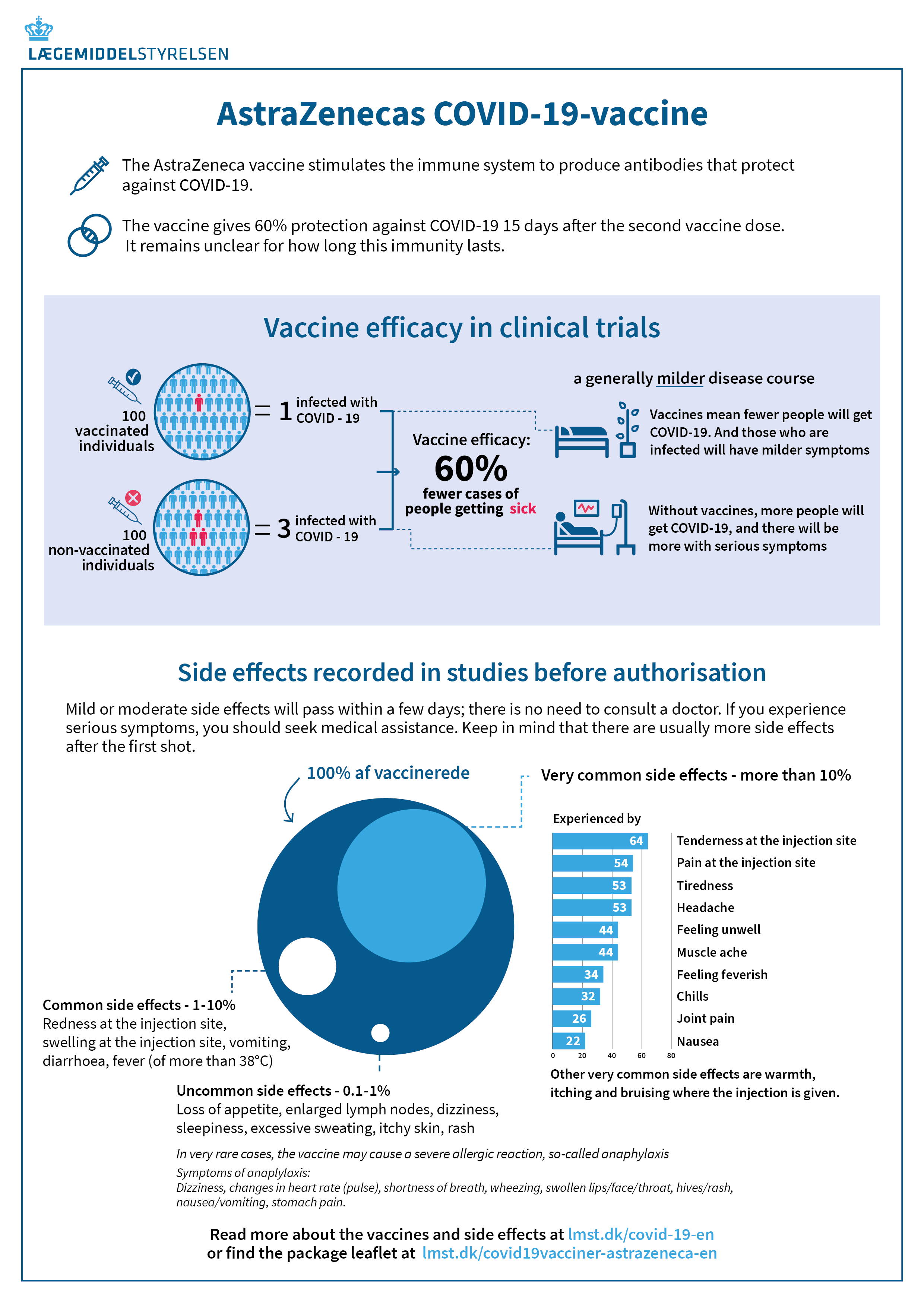
The most common symptoms are mild to moderate, transient side effects such as redness at the injection site, tiredness, headache, muscle and joint pain, chills and fever. When a vaccine activates the immune system, many will experience influenza-like symptoms. This is completely normal and a sign that the body’s immune system is reacting to the vaccine by boosting the body’s defence against COVID-19.
What are the symptoms of low levels of blood platelets and blood clots?
A bruise at the injection site is to be expected, but if you have a low platelet count, this could affect the blood's ability to clot. The symptoms of low levels of blood platelets could be easy bruising, small red spots (bleeding) under the skin, or bleeding that does not stop as normal.
The symptoms of blood clots vary depending on the blood clot’s location in the body. For example, it may be a severe headache, severe stomach pain, coldness in a leg, sudden and unexpected pain in parts of the body, breathing difficulties or paralysis of one side of the body.
How serious are the suspected cases of side effects?
They can be serious, and there have been reports of deaths in both Denmark and the EU. It is being investigated right now if there is a causal connection between the vaccination and the symptoms.
The Danish Medicines Agency information sheet on AstraZenecas COVID-19-vaccine
Links
Link to the press release from the Danish Health Authority: Denmark continues its vaccine rollout without the COVID-19 vaccine from AstraZeneca
Link to the Danish Health Authority’s theme page on the AstraZeneca vaccine (in Danish only)
Links to news from the European Medicines Agency, EMA, on the AstraZeneca vaccine:
AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets
(07 April 2021)
COVID-19 Vaccine AstraZeneca – Update on ongoing evaluation of blood clot cases
(25 March 2021)
COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets
(18 March 2021)
Investigation of COVID-19 Vaccine AstraZeneca and thromboembolic events continues
(16 March 2021)
EMA’s safety committee continues investigation of COVID-19 Vaccine AstraZeneca and thromboembolic events – further update
(15 March 2021)
COVID-19 Vaccine AstraZeneca: PRAC investigating cases of thromboembolic events - vaccine’s benefits currently still outweigh risks - Update
(11 March 2021)
The Vaxzevria vaccine in general
How does the Vaxzevria vaccine work?
In our theme on authorised vaccines, you can read about:
- How the vaccine Vaxevria works
- The effect produced by the vaccine
- The basis leading to a conditional authorisation
- The most common side effects
- Package leaflet and summary of product characteristics
Reported side effects for COVID-19
Under our theme on reported side effects, you can read:
- Number of people who have started vaccination with Vaxzevria
- Number of fully vaccinated persons
- Number of reports of suspected adverse reactions
- Number of reported adverse reactions reported
- What side effects have been reported and age distribution
Link to Reported side effects with the AstraZeneca vaccine (Vaxzevria)
Monitoring of side effects
The Danish Medicines Agency registers, monitors and assesses reports of suspected side effects in Denmark. New vaccines and new medicines are monitored closely. This applies also to the COVID-19 vaccines, including the vaccine from AstraZeneca (Vaxzevria).
In the monitoring of the safety of COVID-19 vaccines, the Danish Medicines Agency collaborates with the drug regulatory authorities in Europe, the European Medicines Agency, EMA, and the World Health Organization, WHO, and more.
Read more about the extensive monitoring of side effects of the vaccines