How is the Danish Medicines Agency funded?
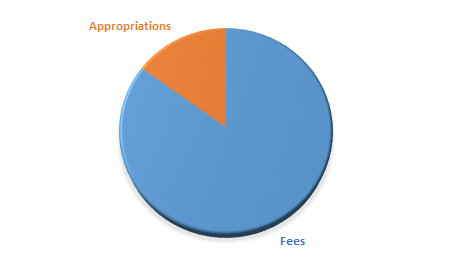
The Danish Medicines Agency is funded partly by appropriations under the state budget and partly by fees and charges paid by participants in the pharmaceutical and medical devices industries.
The balance between fee-based income and state appropriations may vary from year to year, but about 10-15 per cent of our budget is funded by state appropriations, and the remaining 85-90 per cent by fees.
The appropriations under the state budget cover, for example, our costs of administering our reimbursement system, which is the system ensuring that Danish patients can have some of their medicines expenses reimbursed by the state. In the years 2020-2023, the Covid pandemic meant that the Danish Medicines Agency was granted extraordinary appropriations for specific pandemic-related activities.
What does our fee-based income
cover?
- The Danish Medicines Agency's fee-based income covers our costs of the following activities:
- Authorisation of medicinal products for human beings and animals
- Authorisation of clinical investigations and clinical trials
- Monitoring of side effects of medicines
- Maintaining registers for participants in the medical devices area
- Review of reports of incidents and safety corrective actions involving medical devices
- Monitoring of the safety of medical devices
- Planning of the pharmacy structure and supervising pharmacies and the retail sale of medicine
- Review of authorisations for distribution and import of medicines
- Inspection of participants registered in Denmark that manufacture, distribute, import or transport medicines and/or medical devices intended for the Danish and European markets
- Control of the illegal sale of medicines/sale of illegal medicines
The fees are price and wage adjusted every year and each specific fee is the same for all companies, and companies pay exactly the fees laid down by law, no more no less. This means that the fee is the same regardless of the review process and outcome. To illustrate, the fee paid by a company for the submission of an application is the same, whether or not the application results in authorisation. Likewise, a company pays the same fee for an authorisation to manufacture medicines, whether or not an inspection at the factory should lead to identified findings.
Links to further information on fees
Fees payable for medicinal products and companies (laegemiddelstyrelsen.dk)
Fees payable for clinical trials (laegemiddelstyrelsen.dk)
Fees payable for medical devices (page only available in Danish, laegemiddelstyrelsen.dk)
Fees payable by pharmacies (laegemiddelstyrelsen.dk)
Fees payable for national scientific advice (laegemiddelstyrelsen.dk)
Fees paid by retail distributors of medicines (laegemiddelstyrelsen.dk)
Fees in the medicinal cannabis area (laegemiddelstyrelsen.dk)
Fee for reimbursement of nutritional products (laegemiddelstyrelsen.dk)