Brokering – questions and answers
Please note that this website has yet to be updated regarding the regulation (EU) 2019/6 on veterinary medicinal products. We refer to the Danish website for the updated information.
What is the definition of brokering?
Brokering means any form of activities associated with the buying and selling of medicinal products, except for wholesale distribution. Brokering does not include the physical handling of medicinal products, but consists of negotiating independently and on behalf of another legal or natural person.
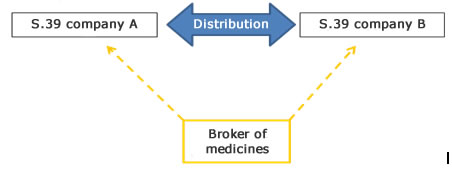
See the Danish executive order on the distribution of medicinal products.
Does brokering include both human and veterinary medicinal products?
Yes. The amendment of the directive only applies to human medicinal products, but there is a tradition in Denmark that new rules also apply to veterinary medicinal products to the extent possible.
Do the rules on brokering also apply to brokering of medicinal products to and between third countries (countries outside the EU/EEA)?
Brokering applies to medicinal products with a marketing authorisation in the EU/EEA. A company brokering medicinal products with a marketing authorisation in the EU/EEA to and/or in third countries must register with the Danish Medicines Agency and is covered by the rules laid down in the Danish GDP executive order for brokers.
Should a Danish company be registered in the countries in which the brokering activities of medicinal products take place?
No. A broker of medicinal products should only register in the country where the company has its registered address.
Will the Danish Medicines Agency make an inspection?
The Danish Medicines Agency may at any time decide to make an inspection. An inspection will be performed in accordance with the provisions of the GDP executive order.
Should I register as a broker if I have an authorisation for wholesale distribution?
An authorisation for wholesale distribution only covers wholesale distribution activities (any form of activities associated with the purchase, sale, receipt, storage and supply of medicinal products within the EU/EEA or associated with the exporting of medicinal products to third countries).
A company carrying out the activities of wholesale distribution and brokering must have an authorisation for wholesale distribution and register as a broker of medicinal products. This also applies if companies have other authorisations, for example authorisations for the manufacturing and retail distribution of medicinal products.
How do I register?
You can find a form for registration and guidelines on registration on the website of the Danish Medicines Agency.
Registration as a broker of medicinal products
Which rules must a broker comply with?
A broker must comply with the rules on brokers laid down in the Danish GDP executive order. See the guidelines on good distribution practice of medicinal products for human use.
Is a salesperson in Denmark, who is employed by a foreign company, a broker or is a Danish subsidiary of a foreign company a broker?
Generally, no. A salesperson employed by a foreign company (company A) and advertising medicinal products in Denmark is not considered an independent party and is not covered by the concept of brokering of medicinal products.
This also applies to subsidiaries advertising medicinal products on behalf of a foreign company.
As long as the salesperson or subsidiary does not carry out wholesale distribution activities (any form of activities associated with the purchase, sale, receipt, storage and supply of medicinal products within the EU/EEA or associated with the exporting of medicinal products to third countries), they do not need to register as a broker or have an authorisation for wholesale distribution in Denmark.
The salesperson or subsidiary in Denmark can take an order and send it to company A, which will not be considered as brokering or wholesale distribution.
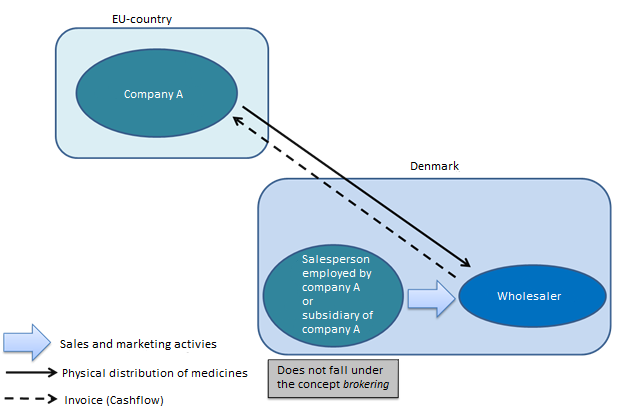
If the salesperson or subsidiary is involved in the cash flow (invoicing – purchase/sale), the scenario changes, because this will be considered an activity for a wholesale distributor.
This requires an authorisation for wholesale distribution in Denmark.
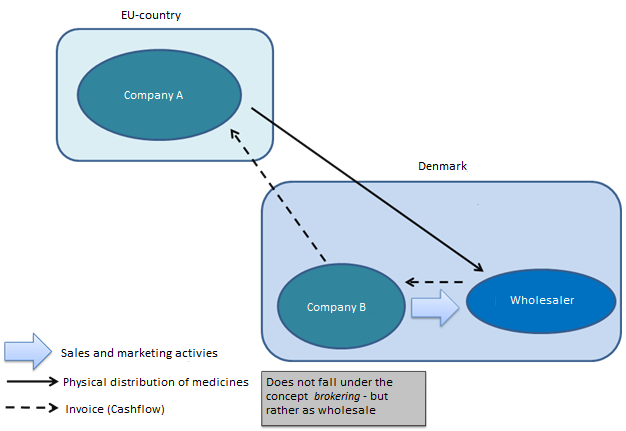
Will a company involved in invoicing but not the physical handling of medicinal products be covered by the concept of brokering or will such company need an authorisation for wholesale distribution?
If a company is involved in the cash flow (invoicing – purchase/sale), this will be considered an activity covered by the concept of wholesale distribution. This requires an authorisation for wholesale distribution in Denmark.
Is a medical sales representative a broker?
A medical sales representative visiting doctors and informing them about medicinal products and advertising medicinal products is not a broker.
A medical sales representative is not considered an independent party and is not covered by the concept of brokering.
