Registration as a broker of medicinal products
Please note that this website has yet to be updated regarding the regulation (EU) 2019/6 on veterinary medicinal products. We refer to the Danish website for the updated information
Here, please find guidelines and forms for registration as a broker of medicinal products.
Companies brokering human and veterinary medicines with a marketing authorisation must register with the Danish Medicines Agency.
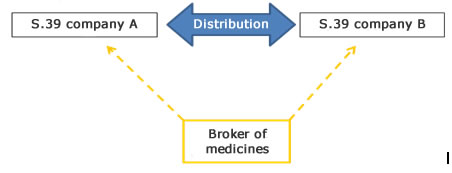
Brokering means activities related to buying and selling (brokering) of medicines not involving the activities of wholesale distribution (purchase, sale, receipt, storage and supply, etc.). Thus, brokering does not include the physical handling of medicinal products, but consists of negotiating independently and on behalf of another legal or natural person.
List of companies having registered as a broker of medicinal products (Excel file)
Illustration of brokering
Companies must register pursuant to section 41B(1) of the Danish Medicines Act, which implements article 85 of Directive 2011/62/EU of 8 June 2011 on falsified medicines. The requirement applies to brokers of medicinal products throughout the EU, but only companies with an registered address in Denmark must register with the Danish Medicines Agency.
Deadlines for registration
As from 1 January 2013, companies are required to register with the Danish Medicines Agency. Companies which started their activities before 1 January 2013 must register with the Danish Medicines Agency by 1 March 2013.
The rules applicable to brokers of medicinal products are established in the Danish executive order on distribution of medicinal products (the GDP order). These rules apply to brokers of both human and veterinary medicines.
Guidelines
Guidelines on activities subject to a section 39 authorisation or company registration
Guidelines on registration as a broker of medicinal products
Form
Form for registration as a broker of medicinal products (Word file)
Inspection
Companies having registered as a broker of medicinal products may be inspected by the Danish Medicines Agency. Inspections will be performed in pursuance of the provisions of the GDP order.