Standardisation of the quality of medicines in Europe
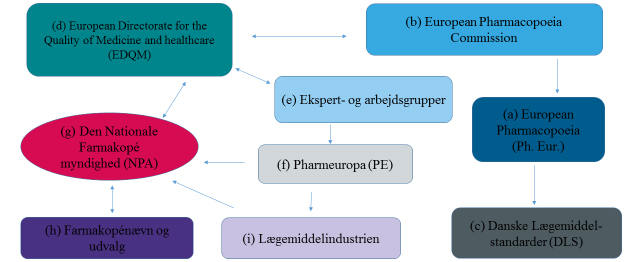
The work to standardise the quality of medicines in Europe revolves around the collaboration on the maintenance and development of the European Pharmacopoeia (Ph. Eur.) (a). Whereas the European collaboration on the licensing of medicines falls under the EU, the collaboration on the quality of medicines falls under the Council of Europe.
The European Pharmacopoeia Commission (b) (Ph. Eur. Commission) is the highest decision-making body. It decides which texts and monographs are to be implemented in or removed from the Ph. Eur. The Ph. Eur. Commission, represented by 38 member states and the EU institution, consists of national delegations with up to three persons from each member state. The commission meets three times a year. In order for the Ph. Eur. to have legal effect in Denmark, it is transposed into Danish law via the Danish Drug Standards (c).
The EDQM (d) is an administrative department/a directorate handling many different functions in relation to the Ph. Eur. collaboration. One of its functions is to provide secretarial support to the Ph. Eur. Commission as well as the various groups of experts and working parties (e) that work with the Ph. Eur. Another important function of the EDQM is to prepare the reference standards related to the methods of analysis in the specific Ph. Eur. monographs. The work of elaborating and revising the current monographs and texts for Ph. Eur. is handled by the various groups of experts and working parties (e).
When new and revised Ph. Eur. texts and monographs have been prepared, they are submitted for commenting in Pharmeuropa (PE) (f), which is published four times a year.
The connecting link between the EDQM, the Danish Medicines Agency and the Danish users of the Ph. Eur. is the National Pharmacopoeia Authority (NPA), which is a staff function in the Danish Medicines Agency. Under the NPA (h), a Pharmacopoeia Commission and four subcommittees have been established. Their job is to support and advise the Danish Medicines Agency in decisions related to the standardisation of the quality of medicines. The main task of the subcommittees is to discuss and prepare joint Danish comments to the proposed texts and monographs submitted for consultation in Pharmeuropa (PE) (f).
The subcommittees prepare comments to the proposed Ph. Eur. texts and monographs together with the Danish Medicines Agency, but also the pharmaceutical industry (i) and other stakeholders can submit comments. All the Danish comments are forwarded to the EDQM via the secretary of the National Pharmacopoeia Authority (NPA).
The National Pharmacopoeia Authority can be contacted by email on Send an email.