Medicine shortages
Denmark generally has a high security of supply in the pharmaceutical area, and people living in Denmark can usually get their prescribed medicines at the pharmacy.
While it sometimes happens that a medicine is temporarily unavailable, it does not necessarily mean returning empty-handed from the pharmacy.
If the medicine you need is not unavailable at the pharmacy, the pharmacy staff is often able to offer it in a different pack size or an alternative medicinal product that corresponds to the one prescribed by your doctor. The medicine might also be available from another pharmacy.
However, in rare cases, the pharmacy has no alternative to offer, which means you will have to contact your doctor again. The pharmacy staff will tell you if this is the case.
Finally, if your doctor finds it necessary, they have the option of applying for a compassionate use permit from the Danish Medicines Agency for the dispensing of a medicine that is not marketed in Denmark. If the Danish Medicines Agency grants the application, you can subsequently pick up the concerned medicine at a pharmacy in Denmark.
Healthcare professionals may also experience periods when certain medicines become unavailable. The Danish Medicines Agency is not allowed to promote products over other products and so offers no guidance on the choice of medicines, so if you need guidance, please contact other healthcare professionals, including pharmacy staff; they can advise you about alternative medicinal products. Please also know that you may apply to the Danish Medicines Agency for a compassionate use permit for medicines not marketed in Demark.
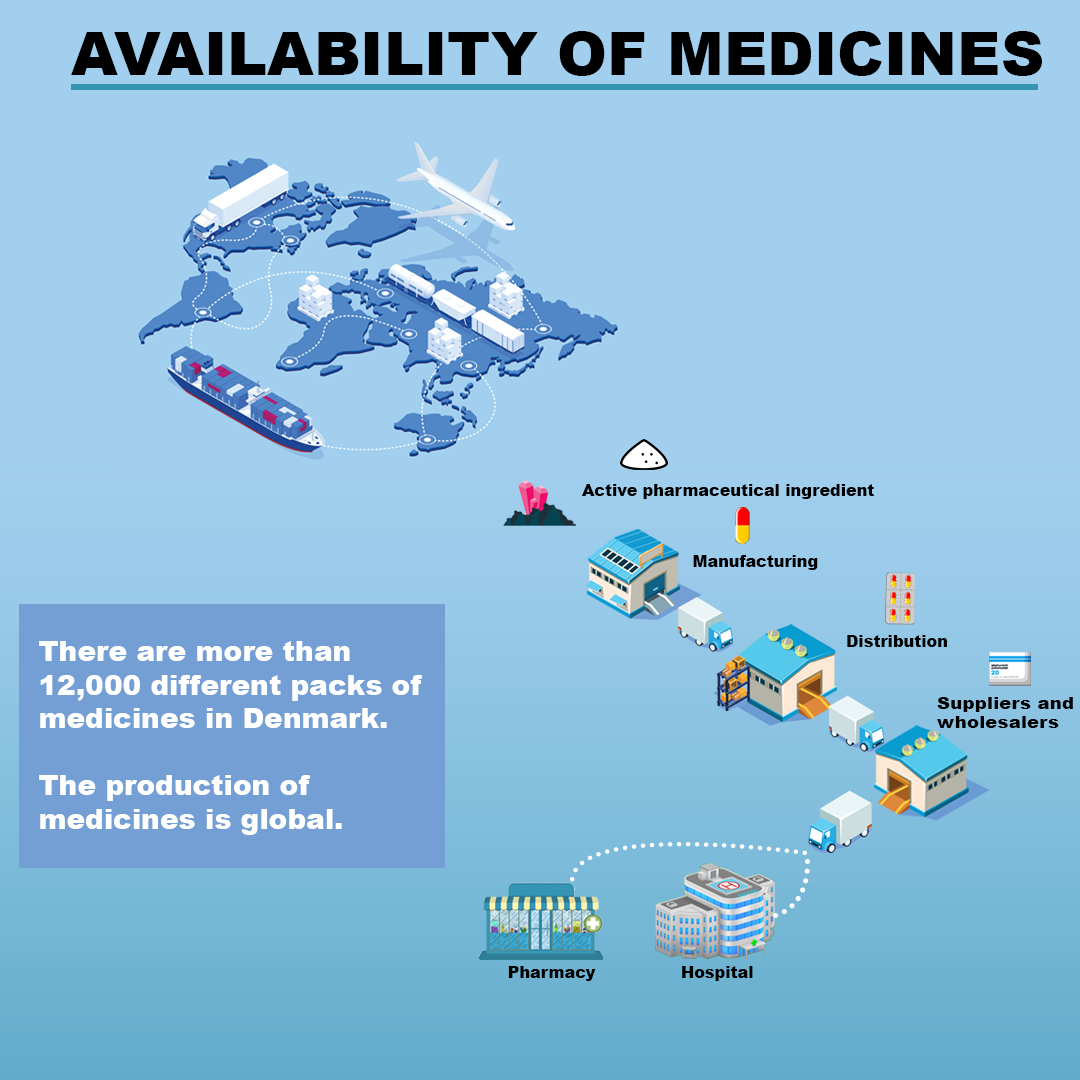
Why do pharmaceutical supply difficulties arise?
Like other products produced in different countries and marketed in Denmark, there could be a number of reasons why a medicine for a period of time cannot be supplied to hospitals or is not available from pharmacies.
Shortages in medicines could be due to
- a lack of the raw material used in the medicine
- flaws in the production quality
- production failures at a supplier
- the medicine being on back order from a distributor
- the product being sold out at the pharmacy
- a higher demand for the medicine than expected by the pharmaceutical company
- the pharmaceutical company assessing that marketing the medicine is no longer worthwhile
National Council for the Security of Supply of Medicines
The Danish Medicines Agency keeps a close watch on the supply situation in Denmark and collaborates with wholesalers, companies, pharmacies, hospital pharmacies, the regions and other authorities, nationally and internationally, to minimise the risk of supply and delivery problems in pharmacies and hospitals.
The Danish Medicines Agency has more specifically joined the circle of relevant actors in the National Council for the Security of Supply of Medicines to create a forum through which to pool common knowledge, maintain a focus on the needed reports on supply difficulties and to collaborate on common solutions in general.
Read more about the National Council for the Security of Supply of Medicines.
International collaboration
The Danish Medicines Agency works with the security of supply on the international scene. The drug regulatory authorities in and outside the Nordic region and Europe increasingly work together to prevent or minimise medicine shortages and to limit the consequences in the event of supply challenges. We do this for example via
- collaboration with the pharmaceutical companies to resolve production and distribution problems
- exchange of information with international partners about alternative sources of supply
- development of strategies to prevent problems in the supply chain, for example by preparing guidelines for the companies on the reporting of shortages