CTIS Helpdesk for non-commercial sponsors
11. oktober 2024
Dedicated CTIS helpdesk for non-commercial sponsors
The Accelerating clinical trials in the EU (ACT EU) initiative has established a dedicated helpdesk, which employs different measures to support non-commercial sponsors in navigating the clinical trial landscape in the EU.Currently, the helpdesk offers tailored technical assistance on CTIS functionalities and addresses questions on regulatory requirements related to the clinical trial lifecycle. The helpdesk may also consult National Competent Authorities to provide support on specific cases.
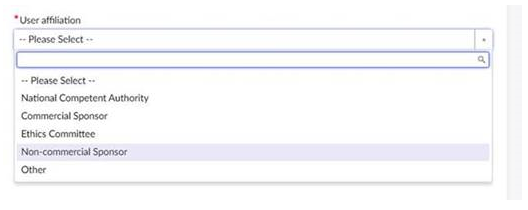
Non-commercial sponsors can submit their question now by raising a ticket in the CTIS Service Desk and indicating their status as a non-commercial sponsor in the mandatory field “User affiliation”.
Link: CTIS Service Desk